
ij��ȤС���������ͼʵ��װ�ý���ʵ�顣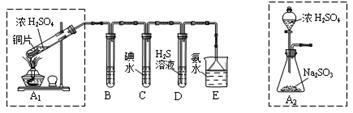
��̽��������Ⱦ��SO2������
��1��Ϊ��ʵ����ɫ������Ŀ�꣬�ܷ�����ͼA2����A1װ�� ����ܡ�����
��2��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��C�з�Ӧ�����ӷ���ʽΪ ��D�з�Ӧ�Ļ�ѧ����ʽΪ ��
��̽��ͭƬ��ŨH2SO4��Ӧ�IJ���
ʵ�������������ͭƬ���渽�ź�ɫ���塣�������ϵ�֪���˺�ɫ������ܺ���CuO��CuS��Cu2S��������CuS��Cu2S��������ϡ���ᣬ�ڿ��������ն�ת��ΪCu2O��SO2����С��ͬѧ�ռ�һ������ɫ���壬������ʵ�鷽��̽����ɷ֣�
��3��������м�������ϴ�Ӹɾ���ʵ�鷽����_____________________________��
��4����ɫ����ijɷ���________________��
�������
�ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ�
��5����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��6����֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32-��HSO3-������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ� ������Һ���д���SO32-�� SO42-�� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� | �� ������Һ���д��� SO32-�� |
| ����3�� �� | �� ������Һ���д��� HSO3-�� |
��1���ܣ�2��Ʒ����Һ SO2��I2��2H2O��SO42-��2 I-��4H+ SO2��2H2S="3S��+" 2H2O��3��ȡ���һ��ϴ��Һ�������μ�����AgNO3��Һ����������������ϴ�Ӹɾ�����4��CuO��CuS��Cu2S
��5��NH3·H2O��SO2��NH4+��HSO3-
��6��ʵ����� Ԥ����������� ����2������1�Σ���������Ʒ����Һ���ٵ������2mol/L���ᣬ�� ��Ʒ����ɫ���������ݡ�����������ܽ⣩�� ����3�����Թ�ȡ������Һ�������е��������1mol/LBa(OH)2��Һ [�����1�Σ���������Ʒ����Һ���ٵ���2-3�Σ����������2mol/L����]���� �����ְ�ɫ��������Ʒ����Һ��ɫ���������ݣ�����
���������������1��������������Ũ���ᷴӦ����ͭ��Ũ���������ȡ�����������巴Ӧ���Կ��ƣ������˻�����Ⱦ����2����Ʒ����Һ��������������Ư���ԣ��õ�ˮ����ˮ�������������Ļ�ԭ�ԣ����������������������ԡ�C�е����ַ���ʽ�ǣ�SO2��I2��2H2O��SO42-��2 I-��4H+��D�з�Ӧ�Ļ�ѧ����ʽΪ��SO2��2H2S="3S��+" 2H2O��3����������ϴ�Ӹɾ���ʵ�鷽���ǣ�ȡ���һ��ϴ��Һ�������μ�����AgNO3��Һ��ϡ������������������֤��ϴ�Ӹɾ�����4����ɫ�����������õ���ɫ��Һ�Ͳ����˵������CuO.������CuS��Cu2S����������һ�֡���������ȫ����CuS,�ڿ��������յõ�������ͭ����Ϊ(2.00g��96g/mol)��1/2��144g/mol=1.5g;��������ȫ����Cu2S,�ڿ��������յõ�������ͭ����Ϊ(2.00g��160g/mol)��144g/mol=1.8g.���ڲ�����Cu2O������1.68g.˵��CuS��Cu2S���С���˺�ɫ����ijɷ���CuO��CuS��Cu2S����5����ˮ�����SO2�ķ�Ӧ�����ӷ���ʽΪ��NH3·H2O��SO2��NH4+��HSO3-����6���������κ����ᷢ����Ӧ���������������壬����������ʹƷ����Һ��ɫ��������Ʒ����Һ����������SO32-�����Թ�ȡ������Һ�������е��������1mol/LBa(OH)2��Һ [�����1�Σ���������Ʒ����Һ���ٵ���2-3�Σ����������2mol/L����]���������ְ�ɫ��������Ʒ����Һ��ɫ���������ݣ�������֤������ HSO3-��
���㣺����ͭƬ��ŨH2SO4��Ӧ�IJ��PSO32-��HSO3-�ļ��顣


 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧϰС���������ͼװ����֤��������Ļ�ѧ���ʡ�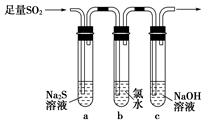
��1����˵������������������Ե�ʵ������Ϊ_________________________��
��2��Ϊ��֤��������Ļ�ԭ�ԣ���ַ�Ӧ��ȡ�Թ�b�е���Һ�ֳ����ݣ��ֱ��������ʵ�顣
���������һ����Һ�м���AgNO3��Һ���а�ɫ��������
��������ڶ�����Һ�м���Ʒ����Һ����ɫ��ȥ
���������������Һ�м���BaCl2��Һ��������ɫ����
���������к�������________������������Թ�b�з�����Ӧ�����ӷ���ʽΪ____________________________________________��
��3����ͨ������������Թ�c����Һ������ʱ������Һ��c��Na������________���ú�����Ũ�ȵĴ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������Ҫ�Ĺ�ҵԭ�ϣ�̽�����Ʊ����������ʾ��зdz���Ҫ�����塣 ��1��Tҵ���û�����(FeS2)�ڸ����º�������Ӧ�Ʊ�SO2��4FeS2��11O2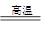 8SO2��2Fe2O3�÷�Ӧ�б�������Ԫ����_______����Ԫ�ط��ţ������÷�Ӧת��2. 75mol����ʱ�����ɵĶ��������ڱ�״���µ����Ϊ_______L��
8SO2��2Fe2O3�÷�Ӧ�б�������Ԫ����_______����Ԫ�ط��ţ������÷�Ӧת��2. 75mol����ʱ�����ɵĶ��������ڱ�״���µ����Ϊ_______L��
��2��ʵ������������װ�òⶨSO2������ΪSO3��ת����(��֪SO3���۵�Ϊ16.8�棬�����������װ��ʱ�ֱ���ȫ���գ��Һ��Կ�����CO2��Ӱ�죩��
�ټ���ʹ�÷�Һ©����Բ����ƿ�еμ�Ũ����IJ�����______��
�ڵ�ֹͣͨ��SO2��Ϩ��ƾ��ƺ���Ҫ����ͨһ��ʱ�����������Ŀ����______��
��ʵ���������װ��D���ӵ�����Ϊmg��װ��E�в�����ɫ����������Ϊng����������¶��������ת������______���ú���ĸ�Ĵ���ʽ��ʾ�����û���
��3��ij��ȤС��������ɫ������������̽��SO2�����ʣ��������ͼʵ��װ�á�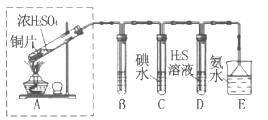
B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ_______��
C�з�Ӧ�����ӷ���ʽΪ______ ��D�е�ʵ������Ϊ______ ��Eװ�õ�������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ���йض����������ʵ�ʵ��װ��ͼ��ʵ��ʱCװ������ˮ��Һ��ɫ���Իش��������⣺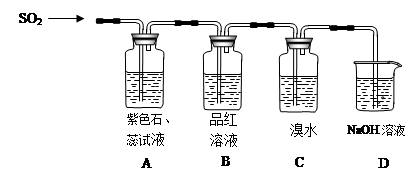
��1��Aװ���е������� �� Bװ���е������� ��
��2������������У���Ư���� ���������������� �ۻ�ԭ�� �������ԣ���ͼ�м���װ���ж�Ӧ���ֳ��������ǣ�A�� ��B�� ��C�� ������ţ���
��3��D��NaOH��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ȤС���������ͼʵ��װ�ý���ʵ�顣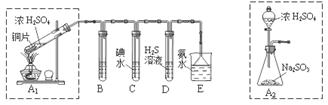
��̽��������Ⱦ��SO2������
��1��Ϊ��ʵ����ɫ������Ŀ�꣬�ܷ�����ͼA2����A1װ�� ����ܡ�����
��2��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��C�з�Ӧ�����ӷ���ʽΪ ��D�з�Ӧ�Ļ�ѧ����ʽΪ ��
��̽��ͭƬ��ŨH2SO4��Ӧ�IJ���
ʵ�������������ͭƬ���渽�ź�ɫ���塣�������ϵ�֪���˺�ɫ������ܺ���CuO��CuS��Cu2S��������CuS��Cu2S��������ϡ���ᣬ�ڿ��������ն�ת��ΪCu2O��SO2����С��ͬѧ�ռ�һ������ɫ���壬������ʵ�鷽��̽����ɷ֣�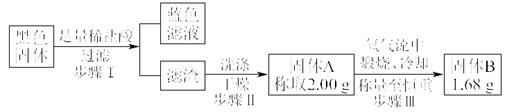
��3������� �м�������ϴ�Ӹɾ���ʵ�鷽����_____________________________��
��4����ɫ����ijɷ���________________��
�������
�ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH����SO32����SO42����HSO3���������ӡ�
��5����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��6����֪����������һ��������ˮ��SO2Ҳ������ˮ��
�����������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ
2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32����HSO3��������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�������Һ�� �д���SO32���� SO42���� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� | �� ������Һ���д��� SO32���� |
| ����3�� �� | �� ������Һ���д��� HSO3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⡣
��1�����з�����,���Ƶ���������ȷ����� ��
��MnO2��Ũ�����Ϲ���
��KMnO4��Ũ������ �������ƺ�Ũ������ ��K2Cr2O7��Ũ������
| A���٢ڢ� | B���٢ڢ� | C���٢� | D��ȫ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������л�ԭ�ԡ���һ�������£�������������ͭ��Ӧ����Ӧ��õ��������ʣ����������ֵ��ʣ���ͼΪ����������ͭ��Ӧ����֤���ֲ����װ��(�г�װ����)��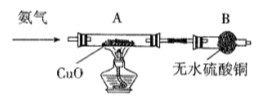
��ش��������⣺
��1��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
��2����֤���������л�ԭ�Ե������� ��
Bװ�õ������� ��
��3��A�з�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ�Ȼ�����һ����Ҫ�Ļ���ԭ�ϣ���������ʯ �Ʊ���ˮ�Ȼ������������£�
�Ʊ���ˮ�Ȼ������������£�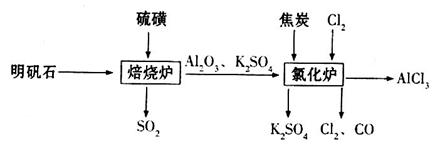
��1����֤����¯���������庬��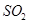 �ķ�����________________________________��
�ķ�����________________________________��
��2�����ձ���¯�в�����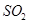 ������װ�ú�������________������ţ���
������װ�ú�������________������ţ���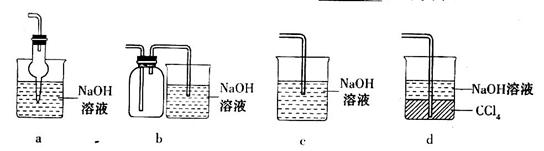
��3���Ȼ�¯�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________��
��4�������Ȼ����Ĺ����в���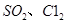 �ȴ�����Ⱦ��������߰���һ������ͨ��ˮ�пɼ��ٻ�������Ⱦ������Ƽ�ʵ���������Ƿ�ǡ����ȫ��Ӧ������Ҫ����ʵ�鲽�衢����ͽ��ۣ�
�ȴ�����Ⱦ��������߰���һ������ͨ��ˮ�пɼ��ٻ�������Ⱦ������Ƽ�ʵ���������Ƿ�ǡ����ȫ��Ӧ������Ҫ����ʵ�鲽�衢����ͽ��ۣ�
__________________________________________________________________________��
������ѡ���ɹ�ѡ���Լ����£�
�ٵμӷ�̪������������Һ ���Ȼ�������Һ �����軯����Һ ��Ʒ����Һ
(5��ijѧϰС�����������װ����֤���������ijЩ��ѧ���ʡ�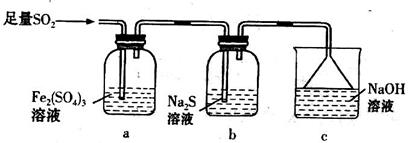
����˵������������������Ե�ʵ������Ϊ_____________________________________��
��д��aƿ�з�����Ӧ�����ӷ���ʽ___________________________________________��
�۳�ַ�Ӧ��ȡaƿ�е���Һ�ֳ����ݣ��ֱ��������ʵ�顣
ʵ��I�����һ����Һ�м���������NaOH��Һ�����ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ
ʵ��II����ڶ�����Һ�м�������KMnO4��Һ����ɫ��ȥ
ʵ��III�����������Һ�м���BaC12��Һ�����ɰ�ɫ����
����ʵ������֤������������л�ԭ�Ե���________����ʵ����ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ���ֽ�����������������������ͼ
��1���ڢܴ������������������ܵ��豸������ ���ô�������Ӧ�ķ���ʽΪ ��Ϊ�����������IJ��ʣ��ô�Ӧ���� ������¹��̡����ȹ��̡���Ϊ�ˡ�
��2���ڢߴ����ж��δ�������ԭ���� ��
��3���ݴ�����������Ҫ�ǵ�������������ʱ���徭�����������������Ϊ
��4��20%�ķ������ᣨSO3����������Ϊ20%��1�����ˮ �֣�����2λ��Ч���֣��������ó�98%�ij�Ʒ���ᡣ
��5���ڢڴ�����1500��ġ�����ȫȼ�ա������Ȼ����������������Ȼ���ڢ۴���700�����ټ���ȼ�ա���˵��Ϊ������ȼ�շ�ʽ�Ի��������������ģ� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com