
 CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��
CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��
 CH3OH+H2O��
CH3OH+H2O��

 CO(g)+2H2(g)�����ݻ�Ϊ2.0L���ܱ������г���0. 60 molCH3OH(g)����ϵѹǿΪP1����һ�������´ﵽƽ��ʱ����ϵѹǿΪP2����P2/P1 =2.2�����������CH3OH ��ƽ��ת����Ϊ______ ��
CO(g)+2H2(g)�����ݻ�Ϊ2.0L���ܱ������г���0. 60 molCH3OH(g)����ϵѹǿΪP1����һ�������´ﵽƽ��ʱ����ϵѹǿΪP2����P2/P1 =2.2�����������CH3OH ��ƽ��ת����Ϊ______ ��
 CH3OH(g) ��H= -91KJ/mol.����ʽ��ӿɵã�CO2(g) +3H2(g)=CH3OH(l)+H2O(l) ��H="-50KJ/mol." �� V(CO2)=" (1.00-0.25)" mol/L��10min=" 0.075mol/(l��min)." V(H2):V(CO2)=3:1,����V(H2)="3" V(CO2)=" 0.225mol/(L��min)" . �ڸ��¶��µ�ƽ�ⳣ����ֵ
CH3OH(g) ��H= -91KJ/mol.����ʽ��ӿɵã�CO2(g) +3H2(g)=CH3OH(l)+H2O(l) ��H="-50KJ/mol." �� V(CO2)=" (1.00-0.25)" mol/L��10min=" 0.075mol/(l��min)." V(H2):V(CO2)=3:1,����V(H2)="3" V(CO2)=" 0.225mol/(L��min)" . �ڸ��¶��µ�ƽ�ⳣ����ֵ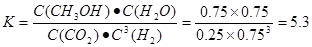 ���ڷ�Ӧ CO2(g) +3H2(g)= CH3OH(l)+H2O(l) ��H=-50KJ/mol.������Ӧ��һ�����ȷ�Ӧ�����Խ����¶���ʹƽ����ϵ��n(CH3OH)/n(CO2)������������ѹ������H2������ˮ�����ӻ�����з�������ȴ�ʩҲ��ʹƽ����ϵ��n(CH3OH)/n(CO2))����3����Ӧ��ʼʱn(CH3OH)=0.6mol,n(CO)=0mol,n(H2)=0mol.���跴Ӧ������CH3OH�ı�����ʵ���ΪX����ﵽƽ��ʱ�����ʵ����ʵ���Ϊn(CH3OH)=" (0.6-X)mol," n(CO) ="Xmol" n(H2)=2Xmol,��������̶����ܱ������е����巴Ӧ��˵����Ӧǰ���ѹǿ�ȵ������ǵ����ʵ����ıȡ�����(0.6+2X)��0.6=2.2,���X=0.36.����CH3OH��ƽ��ת����Ϊ0.36��0.6��100��=60��������ͼ��֪��n(O2)��n(CH3OH) =0.25ʱ�õ��IJ����Ǽ�ȩ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ2CH3OH +O2="2HCHO+" 2H2O�����Ʊ�H2ʱ������n(O2)��n(CH3OH) =0.5ʱѡ������ߣ�������ÿ���n(O2))/n(CH3OH)= 0.5��
���ڷ�Ӧ CO2(g) +3H2(g)= CH3OH(l)+H2O(l) ��H=-50KJ/mol.������Ӧ��һ�����ȷ�Ӧ�����Խ����¶���ʹƽ����ϵ��n(CH3OH)/n(CO2)������������ѹ������H2������ˮ�����ӻ�����з�������ȴ�ʩҲ��ʹƽ����ϵ��n(CH3OH)/n(CO2))����3����Ӧ��ʼʱn(CH3OH)=0.6mol,n(CO)=0mol,n(H2)=0mol.���跴Ӧ������CH3OH�ı�����ʵ���ΪX����ﵽƽ��ʱ�����ʵ����ʵ���Ϊn(CH3OH)=" (0.6-X)mol," n(CO) ="Xmol" n(H2)=2Xmol,��������̶����ܱ������е����巴Ӧ��˵����Ӧǰ���ѹǿ�ȵ������ǵ����ʵ����ıȡ�����(0.6+2X)��0.6=2.2,���X=0.36.����CH3OH��ƽ��ת����Ϊ0.36��0.6��100��=60��������ͼ��֪��n(O2)��n(CH3OH) =0.25ʱ�õ��IJ����Ǽ�ȩ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ2CH3OH +O2="2HCHO+" 2H2O�����Ʊ�H2ʱ������n(O2)��n(CH3OH) =0.5ʱѡ������ߣ�������ÿ���n(O2))/n(CH3OH)= 0.5��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��Ŀ | H2 | N2 | NH3 |
| ��ʼʱ | 5 mol��L��1 | 3 mol��L��1 | 0 |
| 2 sĩ | 2 mol��L��1 | | |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CO(NH2)2(s)+H2O(g)
CO(NH2)2(s)+H2O(g) | ���ʵ���mol | 0min | 2 min | 3 min | 4 min |
| NH3 | 2.0 | 1.4 | n1 | n1 |
| CO2 | 1.0 | 0.7 | n2 | n2 |
| H2O | 0 | 0.3 | n3[ | n3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2NH3(g) ��92.4 kJ������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼʾ������˵���������
2NH3(g) ��92.4 kJ������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼʾ������˵���������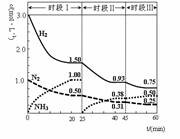
| A��ǰ20���ӷ�Ӧ�ڷų�������Ϊ46.2kJ |
| B����25���Ӹı�������ǽ�NH3�ӷ�Ӧ��ϵ�з����ȥ |
| C������60����ʱ��Ӧ�ִﵽ��ƽ�⣬��ʱ��ı������������ѹǿ |
| D��ʱ�������ʼͶ�ŵ�����Ũ������ԭ����2������Ӧ���ת��������ƽ�ⳣ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2C(g)(����Ӧ����)������ͼ����ȷ����
2C(g)(����Ӧ����)������ͼ����ȷ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2C��g��+ D��s�� ��5 min��ﵽƽ�⡣ƽ��ʱAΪ1.6mol���ų�������ΪQ����t0ʱ�̣�����ƽ����ϵ�з�����ķ�֮һ�Ļ�����壬��ƽ����ϵ��c(A)Ϊ0.6mol��L��
2C��g��+ D��s�� ��5 min��ﵽƽ�⡣ƽ��ʱAΪ1.6mol���ų�������ΪQ����t0ʱ�̣�����ƽ����ϵ�з�����ķ�֮һ�Ļ�����壬��ƽ����ϵ��c(A)Ϊ0.6mol��L��| ��ʼ n��A��/mol | ��ʼ n��B��/mol | ��ʼ n��C��/mol | ��ʼ n��D��/mol | �ﵽƽ��ʱ�ų��������գ������� |
| 0 | 1.6 | 8 | ���� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����ʵ | ���ͻ���� |
| A | 2N2O5(g) 4NO2(g)+O2(g) ��H>0 4NO2(g)+O2(g) ��H>0�����������Է����� | ����һ�������ķ�Ӧ |
| B | ѹ����Ͳ�ڵ�NO2��N2O4������壬 ��ɫ�ȱ�����dz | ����ѹǿ��ƽ��������N2O4�ķ����ƶ�����ƽ��Ⱦ�ƽ��ѹǿС |
| C | п��ϡ���ᷴӦ�����У���ʼ��Ӧ������������ | �÷�Ӧ�Ƿ��ȷ�Ӧ |
| D | �ѽ���ƽ���ij���淴Ӧ�����ı�����ʹ��ѧƽ��������Ӧ�����ƶ� | ��Ӧ���Ũ��һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ�������й�˵��һ����ȷ����
�����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ�������й�˵��һ����ȷ����
| A��������Ӧ�ġ�H>0 |
| B��ѹǿP1>P2>P3>P4 |
| C��1100��÷�Ӧ��ƽ�ⳣ��Ϊ64mol2��L-1 |
| D��ѹǿΪP4ʱ����Y�㣺v(��)<v(��) |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com