
��2011?����ģ�⣩����ѧ�뼼����ģ��
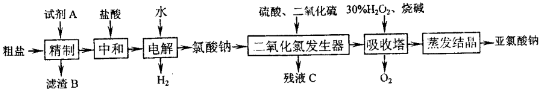
������һ����ı��أ����Ѻ�ˮ�����ͻ�����������������ȿɽ����ˮ��Դȱ�������⣬�ֿɳ�����ú�����Դ�����Ժ�ˮΪ��Ҫԭ�ϵĺ���ѧ��ҵ���ֱ���Ϊ����ɫ��������
��1�����õĺ�ˮ����������
����
����
����
Ĥ
Ĥ
���������������������䶳�������ӽ������ȣ�
��2����ͼ�ǵ�������������ˮ��ԭ��ͼ�����У��缫A��ֱ����Դ���������缫B��ֱ����Դ�ĸ�����
�ٸ�ĤA��
�����ӽ���Ĥ
�����ӽ���Ĥ
��������ӽ���Ĥ�������ӽ���Ĥ��
�ڴ������۲ɼ��ĺ�ˮ��Ʒ�����������д�����Na
+��Cl
-���Լ�������K
+��SO
42-��
��������װ�öԲ��������۵ĺ�ˮ���е�����������������ɺ�A��B��C������������Һ����Һ�壩��pH�ֱ�ΪpH
a��pH
b��
pH
c�������С˳��Ϊ
pHa��pHb��pHc
pHa��pHb��pHc
��
����д���õ��������Բ��������۵ĺ�ˮ���е�������ʱ�������Ļ�ѧ��Ӧ����ʽ
2NaCl+2H
2O
2NaOH+H
2��+Cl
2��
2NaCl+2H
2O
2NaOH+H
2��+Cl
2��
��
��3��������ʱӲ�ȵ�Ӳˮ�ڳ�ʱ�������к����ɳ�������Ҫ�ɷ���
CaCO3��Mg��OH��2
CaCO3��Mg��OH��2
��
��4��Ϊ��ô���ˮ��ȥ����ˮ����ijͬѧ��ʵ���ҽ���Mg
2+��Ca
2+��Cl
-��Ӳˮ�Ⱥ�ͨ�������ӽ�����֬[��RN��CH
3��
3OH]�������ӽ�����֬[��RSO
3H]���Cl
-���������ӽ�����Ӧ�ķ���ʽ
RN��CH3��3OH+Cl-�TRN��CH3��3Cl+OH��-
RN��CH3��3OH+Cl-�TRN��CH3��3Cl+OH��-
�����ʵ��δ��óɹ��������ԭ����
�����ӽ�����֬��������OH-��Mg2+��Ca2+�ȷ�Ӧ���ɳ������������ӽ�����
�����ӽ�����֬��������OH-��Mg2+��Ca2+�ȷ�Ӧ���ɳ������������ӽ�����
��









 +2NaOH
+2NaOH +NaCl+2H2O
+NaCl+2H2O +2NaOH
+2NaOH +NaCl+2H2O
+NaCl+2H2O



 ��2011?����ģ�⣩����ѧ�뼼����ģ��
��2011?����ģ�⣩����ѧ�뼼����ģ��