
����Ŀ��ijѧ��������֪���ʵ���Ũ�ȵ�����(0.1000mol/L)���ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��ѡ�������ָʾ��������д���пհף�
��1���ñ�������ζ������NaOH��Һʱ��������_______�����ʽ����ʽ�����ζ��ܵĻ���������ҡ����ƿ���۾�ע��______________________ֱ���������һ���������Һ�ɻ�ɫ��Ϊ_________ɫ����_________________________Ϊֹ��
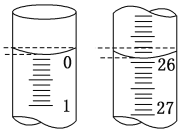
��2���ζ���ʼ�ͽ���ʱ�ζ��ܵ�Һ����ͼ��ʾ�����յ����Ϊ_____mL�������������Ϊ_____mL��
��3�������м��ּ�������������ۣ����ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�����ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и�������ʹ����NaOH��Һ��Ũ����ֵ_________��
������ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�������ʹ����NaOH��Һ��Ũ����ֵ_______��
��4���й����ݼ�¼���£�
�ζ����� | ����Һ���(mL) | �����������mL�� | |
�ζ�ǰ������mL�� | �ζ��������mL�� | ||
��һ�� | 20.00 | 0.50 | 25.40 |
�ڶ��� | 20.00 | 0.00 | 25.10 |
�����������ݣ����������NaOH��Һ��Ũ��Ϊ__________________________________��
���𰸡���ʽ ��ƿ����Һ��ɫ�仯 �� ������ڲ���ɫ 25.90 25.90 ��Ӱ�� ƫ�� 0.1250mol/L
��������
��1����������к͵ζ��Ĺ淶�Բ������⣻
��2�����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��3������c(��) =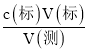 ��������������V(��)����Ӱ�죬�Դ��ж�Ũ�ȵ���
��������������V(��)����Ӱ�죬�Դ��ж�Ũ�ȵ���
��4������ͼ���������1��2��ƽ������V(����)���ٸ��������NaOH��Ӧ���c(NaOH)��
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��2����ʼ����Ϊ0.00mL���յ����Ϊ25.90mL��������Һ�����Ϊ(25.90-0.00)=25.90 mL��
��3�������ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и�������Һ�����ʵ������䣬��V(��)��Ӱ�죬��ʹ����NaOH��Һ��Ũ����ֵ���䣻
������ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V(��)ƫ�������c(��)= 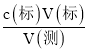 ��֪��ʹ�ⶨ���ƫ�ߣ�
��֪��ʹ�ⶨ���ƫ�ߣ�
��4�����ı�Һ���V(��)=![]() =0.0250L��c(��) =
=0.0250L��c(��) = 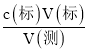 =
=![]() =0.1250mol/L��
=0.1250mol/L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ijԪ�صļ����άͼ������X��һ��ǿ�GΪ���Σ�ͨ��������Z����ɫҺ�壬D�����ԭ��������CС16��������ת����ϵ��ͼ��ʾ������˵����ȷ����( )
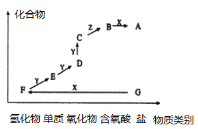
A.D����һ�����ſ������ռ�B.F��Y��һ�������²���ֱ������C
C.A��ˮ��Һ������D.E��D�����ڼ���D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ������Tԭ�ӵ�M���������K���2����������������ȷ���ǣ� ��
![]()
A. �⻯����ȶ��ԣ�R��Q��T
B. T�ĵ�����һ�����õİ뵼�����
C. Q��R�ļ��⻯���������������������������ԭ����ͬ
D. T��W������������ˮ���������Wǿ��T
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����H2(g)��![]() O2(g)=H2O(g) ��H1��akJ��mol1
O2(g)=H2O(g) ��H1��akJ��mol1
��2H2(g)��O2(g)=2H2O(g) ��H2��bkJ��mol1
��H2(g)��![]() O2(g)=H2O(l) ��H3��ckJ��mol1
O2(g)=H2O(l) ��H3��ckJ��mol1
��2H2(g)��O2(g)=2H2O(l) ��H4��d kJ��mol1
���й�ϵʽ����ȷ����
A.a<c<0B.b>d>0C.2a��b<0D.2c��d>0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��������ͼװ�ý�������ˮ������Ӧ��ʵ�飬�������������ʣ���ش��������⣺

(1)Aװ�õ�������____________����ƿ�ײ������Ƭ��������_________________________��
(2)װ��B�з�����Ӧ�Ļ�ѧ����ʽ��____________________________________���÷�Ӧ����������__________������������__________________��
(3)D��������__________________________________��
(4)E�е�ʵ��������____________________________��
(5)A��B����װ����Ӧ�ȵ�ȼ________________���ľƾ�(��)�ƣ���ȼE���ƾ���֮ǰӦ���еIJ�����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������A��D���鷴Ӧ�У���͢����ͬһ�����ӷ���ʽ��ʾ���ǣ� ��
ѡ�� | �� | �� |
A | �ѽ���������ϡ������ | �ѽ���������ϡ������ |
B | �Ȼ�����Һ�м���������NaOH��Һ | NaOH��Һ�м����������Ȼ�����Һ |
C | ϡ�����м�������������������Һ | ����������Һ�м���������ϡ���� |
D | ������Na2CO3��Һ��������HCl��Һ�� | ������HCl��Һ��������Na2CO3��Һ�� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС��ģ���������Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ�ͼ1��ʾװ����ȡNaHCO3����Ӧ�Ļ�ѧ����ʽΪNH3��CO2��H2O��NaCl===NaHCO3����NH4Cl��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��

��1��װ���ҵ�������______________________________________��Ϊ��ֹ��Ⱦ������β���к��е�______________��Ҫ�������մ�����
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������________________��ϴ�ӡ����ա�
��3������(2)�����յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1min��NaHCO3��Ʒ����ɽ������о���ȡ������t1min��NaHCO3��Ʒ29.6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��裬��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ2��ʾ��������c��Ӧ����Һ�е�������______________________(�����ӷ���)������Ʒ��NaHCO3��Na2CO3�����ʵ���֮����__________��
��4����ȡ10.5 g NaHCO3���壬������t1min��ʣ����������Ϊ7.4 g������Ѵ�ʣ�����ȫ�����뵽200 mL 1 mol��L��1�������У����ַ�Ӧ����Һ��H�������ʵ���Ũ��Ϊ__________(����Һ����仯���Բ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͭ���仯����Ӧ�÷dz��㷺�������й�˵����ȷ���ǣ� ��
A.���¸ֹܳ��������������������������������Ͽ���ͭ
B.��ӦCu2S+O2![]() 2Cu+SO2��ÿ����1molCuת�Ƶ�����Ϊ6.02��1023
2Cu+SO2��ÿ����1molCuת�Ƶ�����Ϊ6.02��1023
C.��Ӧ2Cu+CO2+H2O+O2=Cu2(OH)2CO3���������Է����У��÷�Ӧ����H<0
D.�����£�Ksp[Fe(OH)3]=4��10-38��pH=4�ĺ�Fe3+��Һ�У�c(Fe3+)��4��10-8mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ZulemaBorjas����Ƶ�һ���������γص�װ����ͼ��ʾ������˵����ȷ���ǣ� ��
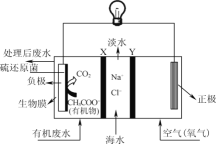
A.��װ�ù���ʱ������ת��Ϊ��ѧ��
B.��װ�ÿ����ڸ����¹���
C.XΪ�����ӽ���Ĥ��YΪ�����ӽ���Ĥ
D.������ӦΪCH3COO-+2H2O-8e-=2CO2��+7H+
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com