
���������(NH4)2Fe(SO4)2��6H2O��һ��dz��ɫ���壬��ˮ�е��ܽ�Ƚ�С���������Ҵ���ijʵ��С�����ö�п��Ƭ���Ʊ���������淋Ĺ������£�
��1������������Ϊ�˳�ȥп�Ʋ㣬�ж�п�Ʋ��ѱ���ȥ�������� ��
��2��A���ʿ����� ��ѡ����ţ���
a��CuCl2 b��CuO c��Cu(NO3)2 d��CuSO4
��������A���ʵ�Ŀ���� ��
��3�������������Ϊ ��
��4���������ɶ����ü��Ⱥ�ɾ����ԭ���� ��
��5����ҵ�ϳ���K2Cr2O7��Һ�ⶨ��������淋Ĵ��ȣ���Ӧ��Cr2O72������ԭ��Cr3+��
д�������������Һ������K2Cr2O7��Һ��Ӧ�����ӷ���ʽ ��
16����12�֣�
��1����Ӧ����ͻȻ��С������Ƭ�������ɵ�����ͻȻ���٣���˼��������֣���2�֣�
��2��b��d��2�֣����1����1�֣����2����2�֣����������֣�
�ӿ�����ϡ����ķ�Ӧ���ʣ�2�֣�
��3�����ˣ�2�֣�
��4��������ȹ����о������ȷֽ�ʧȥ�ᾧˮ����������2�֣�
��5��6Fe2+��Cr2O72����14H+��2Cr3+��6Fe3+��7H2O��2�֣�
�������������
��1����п��Ƭ���������п����γ�Zn-HCl-Feԭ��أ������ܼӿ�H+�ķ�Ӧ���ʣ���������������ͻȻ���١�
��2�������A������Ҫ��Ϊ������Ӧ�ӿ����ʣ�ͨ������Fe-H2SO4-Cuԭ�����ʵ�֣�������Һ��Ҫ����FeSO4������a��CuCl2����ѡ�����ջ���FeCl2���ʣ�c��Cu(NO3)2 �����NO3-���ӣ���������ж������������һ��������Cu(NO3)2��ֻ��b��CuO ��d��CuSO4��������������ܼӿ췴Ӧ���ʡ�
��3�������������Ϊ���ˣ���ȥδ��Ӧ�������ͭ��
��4������Ŀ������(NH4)2Fe(SO4)2��6H2O�еļ��нᾧˮ��ͬʱ��Ϊ+2 �ۣ������ü��Ⱥ�ɿ��ܻ�ʧȥ�ᾧˮ�ͼӿ�+2 �۵�������
��5������������ԭ���ɣ�һ����Ӧ����Ԫ�ػ������ߣ���Ȼ�н��͵ģ���Ŀ�Ѿ�������Cr2O72������ԭ��Cr3+Ԫ�ػ��ϼ��ǽ��͵ģ������������Һ��ֻ��Fe2+���ϼ����ߵ�+3�ۣ��ȸ��ݵ�ʧ�������д��6Fe2+��Cr2O72����2Cr3+��6Fe3+���ٸ�����Һ������ͨ������غ���ɷ���ʽȱ����ƽ�����H+���ڱ�H2O��
���㣺�����Թ�������Ϊ��������Ԫ�ؼ��������ѧʵ�����������ԭ���ԭ����������ԭ����ʽ��ƽ�����֪ʶ��


 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ������0.1 mol/LBa(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2�Լ���������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���
��1�����²��ձ���δ����ΪBaCO3��������_____________�����鷽����_______________��
��2��Ϊȷ�ⶨ��Ʒ��Ba(OH)2�ĺ�������������ʵ�飺
������250 mLԼ0.1 mol/L Ba(OH)2��Һ����ȡ5.000 g�����������ձ��У�����������ˮ����ֽ����ܽ⣬���ù��ˣ�����Һ�ͳ���������Һת��______�У�ϴ�ӣ����ݣ�ҡ�ȡ�
�ڵζ���ȷ��ȡ25.00 mL������Ba(OH)2��Һ����ƿ�У��μ�2�μ��ȣ���0.200 mol/L������װ��ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ��������24.20 mL��������Ʒ��Ba(OH)2����������Ϊ____________��
��3�������ζ��У��ζ�����ע�������֮ǰ����������ˮϴ��������________________���ڵζ��У�ȷ����Ӧ���ǵζ��������ߵ�________________������Ӧ�Ŀ̶ȡ��ζ��յ��������_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ȸʯ��Ҫ��Cu2(OH)2CO3����������Fe���������SiO2��ʵ�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O��CaCO3���������£� 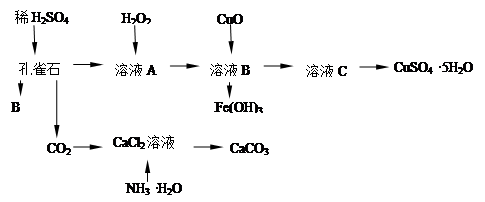
�Իش��������⣺
��1��B���ʵĻ�ѧʽ�� ��δ����H2O2����Һ�У����ڵĽ���������Cu2+��Fe2+��Fe3+�����������Һ��Fe3+��ѡ������ʵ��Լ��� ������ţ���
| A��KMnO4��Һ | B��Fe�� | C��Na2CO3��Һ | D��KSCN��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼��ƺ�����ƶ��ǸƵ���Ҫ��������������������ж����Ź㷺��Ӧ�á��ס�������ͬѧ�ֱ��̼��Ƶ��Ʊ�������Ƶ����ʽ���������̽����������벢��ɶ��й�����Ľ��
(1)����ʹ�ô���ʯ(��������Fe2O3����)�������Ʊ�̼��Ƶ�ʵ���������£�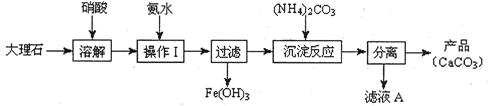
���ܽ����ʯʱ������������������ԭ���� ��
�����������У������롱�ò�Ʒ��������ʵ���������Ϊ�����ˡ� �� ��
�ۡ���ҺA���г�H+�����⣬�����е��������� ������������ӵ�ʵ�鷽���ǣ�ȡ������ҺA�� ���Թ��л�ϡ����ȳ�ַ�Ӧ����ʪ��ĺ�ɫʯ����ֽ(��pH��ֽ)�����Թܿڣ��۲����ɡ�
(2)�����ij����ƾ���(xCaS04��yH20)���ȷֽ���йط�Ӧ����̽��������ȡ6.52g�þ�����м��ȣ����ȹ����У�����������ʱ��ı仯�������ͼ��ʾ����֪t5��t6ʱ����ڹ������������ԭ���Dz������������壬��Ӧ�Ļ�ѧ����ʽΪ��
2CasO4  2CaO+2S02��+O2����
2CaO+2S02��+O2����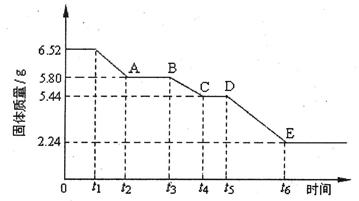
�ټ���ʱ���þ��忪ʼ������ѧ�仯��ʱ���� (�t1������t3����t5��)��
��t4��t5ʱ��ι���Ļ�ѧʽΪ ��
��tl��t2ʱ��ι��巢����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���Ȼ���(SCl2)�۵�-78�棬�е�59�棬�ܶ�1��638g��mL����ˮ�ֽ⣬���Ȼ����������������ÿ�������Ҫ�����Լ���������(SOCl2)����������������ϳɶ��Ȼ����ʵ��װ�á�
�Իش��������⣺
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��װ��B��CӦʢ�ŵ�ҩƷ�ֱ��� �� ��
��3��ʵ�鿪ʼǰ����D�з�һ��������ۣ�����ʹ���ۻ���Ȼ��ת����ҡ����ƿʹ��������ƿ�ڱ��γ�һ������棬��������Ŀ���� ��
��4��ʵ��ʱ��Dװ���������50��59�森��ò��õĴ�ʩ�� ����η�ֹE��Һ��ӷ��� ��
��5��Fװ���и��������ʢ������ �������� ��
��6���ɶ��Ȼ�����SO3���������������ȵĻ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Aװ����װ�е���ɫ�Ĺ��壬��Һ©����װ��Ũ���ᣬB��ʢŨ���ᣬC�з��д�����D��ʢ���۵⻯����Һ��E��ʢ������NaOH��Һ��F��ʢFeSO4��H2SO4�����Һ�� 
�ȴ�ֹˮ�У�ͨ��N2����װ���п������Ͼ����ڼֹˮ�У���ȼ�ƾ��ƣ��ӷ�Һ©������Ũ���ᣬD����ҺѸ�ٱ�����F����Һ��dz��ɫ��Ϊ�ػ�ɫ(����װ�ù�O3)��
��1������ɫ����������ѧ��������Ϊ��________��װ���ڻ�ɫ�Ĺ�������������_______��
��2����μ��װ�õ�������___________________________________________________��
��3��C�з�Ӧ�Ļ�ѧ����ʽ___________________________________________________��
��4��Eװ���з�Ӧ�����ӷ���ʽ_______________________________________________��
��5����F������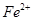 ����μ��麬��Fe2+____________________________________��
����μ��麬��Fe2+____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ظ����ƣ�Na2Cr2O7��2H2O���׳ƺ췯�ƣ���һ����Ҫ�����β�Ʒ����������������ӡȾ�����ϡ���ơ�ҽҩ�ȹ�ҵ�����й㷺����;��ij��ѧ��ȤС�����ʵ��ⶨ�г��ϵĺ췯����Na2Cr2O7�������������䲽�����£�
I����ȡWg�췯�ƣ����������l00mL��Һ��
����cmol��L-1�ı�KMnO4������Һ�ζ�20.00mLһ��Ũ�ȵ�FeSO4��Һ������KMnO4��Һ20.00rnL��
��ȡ20.00mL Na2Cr2O7��Һ��������FeSO4��Һ�ζ����ﵽ�ζ��յ�ʱ������24.00mLFeSO4��Һ��
��������֪��Cr2O72-�����������¾���ǿ�����ԣ��ױ���ԭΪCr3+
�ش��������⣺
��1��Na2Cr2O7��FeSO4�ķ�Ӧ���ӷ���ʽΪ .
��2��������еĵζ����̣��ζ��յ������Ϊ ��ѡ�� �����ʽ����ʽ�����ζ��ܡ�
��3����֪��MnO4-+5Fe2++8H+==Mn2++5Fe3++4H2O���˺췯����Na2Cr2O7�����������ı���ʽΪ ��
��4���ж����в�����Na2Cr2O7�����������ⶨ�����Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�ٲ�����еĵζ����̣��ζ��յ����ʱ���Ӷ��� ��
������c mol��L-1��KMnO4����Һʱ��ת��ʱ��������Һ���� ��
��5��[ʵ��̽��]��ͬѧ���FeSO4�к�ǿ�Ļ�ԭ�ԣ��ڿ������ױ����������ʣ���˶Բⶨ��������Ӱ�죬���������ָ�������FeSO4���ʣ��Բⶨ�����Ӱ���� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�����һ����ʵ�����FeSO4��Һ�Ƿ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���г�ȥ���ʵķ�������ȷ����
| A�����к��б������ʣ�������ˮ������ |
| B���Ҵ��к����������ʣ�����̼������Һ����Һ |
| C��FeCl3��Һ�к���CuCl2���ʣ�����������ۣ����� |
| D��CO2�к���HCl���ʣ�ͨ�뱥��NaHCO3��Һ��ϴ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com