
����Ŀ����Ҫ�����:
(1)ijͬѧ����������ԭ��Ӧ��2Ag++Cu=Cu2++2Ag��Ƶ�ԭ�����ͼ��ʾ��
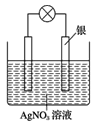
�ٸ��������ĵ缫��ӦΪ___________��
�ڵ������Һ�е�NO3-��__________�缫�ƶ���(д���缫���ϵ�����)
(2)����Ӧ���е�һ��ʱ���ȡ���缫���ϣ����ijһ�缫������5.4g�����ԭ��ط�Ӧ��ת�Ƶĵ�����Ŀ��________��
(3)ˮ������֮Դ��Ҳ�ǻ�ѧ��Ӧ�е����ǡ���ش��������⣺
��֪��2mol H2��ȫȼ������Һ̬ˮʱ�ų�572 kJ��������
����2mol������ȫȼ������ˮ��������ų�������______(����>����<������=��)572 kJ��
��ÿ������ȼ������Һ̬ˮʱ�ų�������Ϊ________��
(4)��Ȼ��(��Ҫ�ɷ�CH4)��������Ӧ���ɶ�����̼��ˮ���÷�ӦΪ���ȵ�������ԭ��Ӧ���ɽ�����Ƴ�ȼ�ϵ�أ�������ͼ��ʾ��a��b�����缫���ɶ��̼����ɡ�
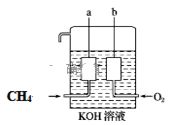
a�缫�ĵ缫��Ӧʽ��_________��
���𰸡�Cu2e=Cu2+ ͭ 0.05NA(��3.01��1022) < 143kJ CH48e+10OH=CO32-+7H2O
��������
(1)�ٸ��ݵ�ط�Ӧʽ֪��ʧ���ӻ��ϼ����ߵĽ�������������������������Ӧ��
����Һ�е�������������ɽ϶�ĸ����ƶ���
(2)���ݵ缫��Ӧʽ����ͨ�����ӵ����ʵ�����
(3)����ͬ������ͬһ���ʣ����庬�е�������Һ̬�࣬Һ̬�ȹ�̬������жϣ�
�ڼ���2mol������������Ȼ����ݷ�Ӧ��������뷴Ӧ�ų������������ȼ���ų��������٣�
(4)ͨ��ȼ�ϵĵ缫Ϊ������ʧȥ���ӣ�����������Ӧ����Ӧ����Ҫ��ϵ������Һ������Է�����
(1)�ٸ��ݷ�Ӧ2Ag++Cu=Cu2++2Ag��֪��Cuʧȥ���ӣ���Ӧ������Ӧ�������ԭ��صĸ������缫��ӦʽΪCu2e=Cu2+��
�ڸ���ͬ�ֵ����ų⣬���ֵ���������ԭ����Һ�е�NO3-��������ɽ϶�ĸ���Cu�缫�����ƶ���������������Ӧ��Ag++e-=Ag��n(Ag)=n(e-)=5.4g��108g/mol=0.05mol�����Ե���ת����ĿΪ0.05NA��
(3)��2mol H2��ȫȼ������Һ̬ˮʱ�ų�572 kJ����������2mol H2��ȫȼ��������̬ˮ�����ڵ�������ˮ�������е�������Һ̬ˮ�ߣ���˷�Ӧ�ų���������572 kJ�������٣�
��2mol H2��ȫȼ������Һ̬ˮʱ�ų�572 kJ��������2molH2������Ϊ4g����1g H2��ȫȼ������Һ̬ˮʱ�ų�������Ϊ572 kJ��4=143kJ��
(4)����ͼʾ��֪a�缫��ͨ��ȼ�ϵĵ缫��Ϊ����������ʧȥ���ӣ����ڵ������ҺΪKOH��Һ���Լ��ԣ�����a�缫�ĵ缫��Ӧʽ��CH48e-+10OH=CO32-+7H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̬��D���ܶ�Ϊ1.16 g��L��1(��״��)����������ת����ϵ�ƶϣ�A![]() B
B![]() C��A
C��A![]() D
D![]() E��
E��
(1)д��A��E�Ľṹ��ʽ��
A��________________��B.________________��C��________________��D.________________��
E��________________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
AB��________________________________________________________________________��
BC��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��a�з��ø�������ͭ��ĩ��ʯ���ޣ���a�г���ͨ����̬����X�����Թ۲쵽ʯ�����Ϻ�ɫ��ĩ��ɺ�ɫ��̬���ʣ�ͬʱc����U�ι�������ɫҺ������(����X����ȫ����Ӧ��������Ӧ����ȫ)��
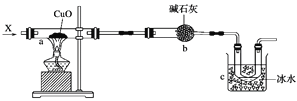
(1)д��a����Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
(2)c��Һ����Ҫ�ɷֵĽṹ��ʽΪ__________________________����������ʵIJ���������_________________________________��������Ϊ______________________________���йط�Ӧ�Ļ�ѧ����ʽΪ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̽���һ�ֺϳ�·�����£�

��֪��
I.![]()
II.![]()
III.RCOOH+CH![]() CH
CH![]() RCOOCH=CH2
RCOOCH=CH2
�ش���������
(1)A��������______��C�������������ŵ�������_______��C��D�ķ�Ӧ������________��
(2)B��������Һ��Ӧ�����ӷ���ʽΪ_______________��
(3)D+G����̽��Ļ�ѧ����ʽΪ_________________��
(4)T��C��ͬ���칹�壬T�����������ʻ����������ܷ���ˮ�ⷴӦ��������Ӧ������ʹ������Ȼ�̼��ɫ�������ڷ����廯�����T�Ľṹ��____�֡����к˴Ź�������Ϊ5��壬�ҷ������Ϊ1��1��2��2��2�Ľṹ��ʽΪ_______��
(5)��������֪ʶ����������Ϣ��д����CH3CH2OHΪԭ���Ʊ�CH3CH2CH2COOH�ĺϳ�·������ͼ_______(���Լ�����)��
���ϳ�·������ͼʾ�����£�CH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2OH��
CH3CH2OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ���ǣ� ��
A. �������ն��������⻯ѧ�������������������ꡱ���γɶ��뵪���������й�
B. ��ҵ��ˮ��ȡþ���̣���ˮ![]() Mg(OH)2��MgCl2
Mg(OH)2��MgCl2![]() Mg
Mg
C. �ƹ�ʹ���Ҵ����ʹ�������Ŀ����Ϊ�˼�������������ŷ�
D. ��ҵ����������ˮ���ʯ��ʯ��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ͼ���Ӧ���ϵ���(����)

A. ���ڴﵽƽ��״̬��N2(g)��3H2(g)![]() 2NH3(g)��t0ʱ�̳�����һ����NH3��ƽ�������ƶ�
2NH3(g)��t0ʱ�̳�����һ����NH3��ƽ�������ƶ�
B. P2>P1��T1>T2
C. ��ͼ���ʾ�ķ���ʽΪ��2A===B��3C
D. ���ڷ�Ӧ2X(g)��3Y(g)![]() 2Z(g)����H<0��y���Ա�ʾY�İٷֺ���
2Z(g)����H<0��y���Ա�ʾY�İٷֺ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ���ѡ����ȷ���ǣ� ��
A. 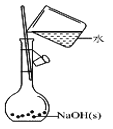 ����0.10mol/LNaOH��Һ
����0.10mol/LNaOH��Һ
B. 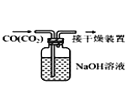 ��ȥCO�е�CO2
��ȥCO�е�CO2
C. 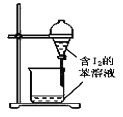 ����ȡ��ˮ�е�I2���ֳ�ˮ���IJ���
����ȡ��ˮ�е�I2���ֳ�ˮ���IJ���
D. 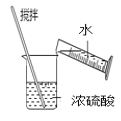 ϡ��Ũ����
ϡ��Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ӻ˴Ź����ף�PMR�����о��л���ṹ�������ֶ�֮һ�������о��Ļ���������У�ÿһ�ṹ�еĵ�����ԭ����PMR���ж���������Ӧ�ķ壨�źţ������з��ǿ����ṹ�е�Hԭ���������ȡ����磺��ȩ�ĽṹʽΪ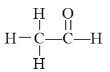 ������PMR������2���źŷ壬��ǿ��֮��Ϊ3��1��
������PMR������2���źŷ壬��ǿ��֮��Ϊ3��1��
��1������ʽΪC3H6O2�Ķ�Ԫ��������PMR���Ϲ۲쵽��ԭ�Ӹ����ķ��������������һ��������ǿ��֮��Ϊ3��3���ڶ���������ǿ��֮��Ϊ3��2��1���ɴ��ƶϿ�����ɸû����ĸ������ǣ�д�ṹ��ʽ����__________________��__________��_______________��
��2���ڲ�õ�PMR���Ͽɹ۲쵽������![]() ��3���壬��CH3CH��CHCl������ȴ�õ���ԭ�Ӹ�����6���źŷ塣����ԭ���ڿռ�����з�ʽ�IJ�ͬ��д��CH3CH��CHCl���ӵĿռ��칹�壺_____________________________________��
��3���壬��CH3CH��CHCl������ȴ�õ���ԭ�Ӹ�����6���źŷ塣����ԭ���ڿռ�����з�ʽ�IJ�ͬ��д��CH3CH��CHCl���ӵĿռ��칹�壺_____________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com