
����Ŀ��25 ��ʱ����Ũ�Ⱦ�Ϊ0.1 mol��L��1��NaOH��Һ������ֱ�ζ������Ϊ20 mLŨ�Ⱦ�Ϊ0.1 mol��L��1��HA��Һ��BOH��Һ���ζ���������Һ��pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
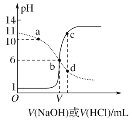
A.HAΪ���ᣬBOHΪǿ��
B.a��ʱ����Һ������Ũ�ȴ��ڹ�ϵ��c��BOH��<c��B����
C.b��ʱV��20
D.c��d������Һ��Ϻ���֮����ڹ�ϵ��c��H������c��OH������c��BOH��
���𰸡�D
��������
����ͼ�����������ͼ����HCl�ζ�BOH��Һ��ʵ��ͼ����NaOH�ζ�HA��Һ��HA��Һ��BOH��Һ��Ϊ0.1mol/L��������ʼ״̬�жϣ�HA��Һ��pHΪ1����HA��ȫ���룬��ǿ�ᣬBOH��ʼʱ��Һ��pHΪ11������ȫ���룬��BOH����������Һ�е��غ�˼�룬�ݴ��жϷ�����
����ͼ�����������ͼ����HCl�ζ�BOH��Һ��ʵ��ͼ����NaOH�ζ�HA��Һ��HA��Һ��BOH��Һ��Ϊ0.1mol/L��������ʼ״̬�жϣ�HA��Һ��pHΪ1����HA��ȫ���룬��ǿ�ᣬBOH��ʼʱ��Һ��pHΪ11������ȫ���룬��BOH�����
A��HA��ǿ�ᣬBOH�������A����
B��a������������0.1mol/L��HCl�ζ�20.00mL0.1mol/L��BOH��������Һ�еĵ���غ㣬c(H+)+c(B+)=c(OH)+c(Cl)����ҺΪ���ԣ���������BOH��c(BOH)> c(B+)����B����
C��b��ʱ������ͼ��ʱ��ҺpHֵΪ6����ҺΪ���ԣ�����ʱV=20������0.1mol/L��NaOH�ζ�20.00mL0.1mol/L��HA��Һ��˵����ʱΪ�ζ��յ㣬ǡ������NaA������HAΪǿ�ᣬ��ΪNaA������ˮ�⣬��ʱӦΪ���ԣ�����V=20�����ϣ���C����
D��c�����NaOH�ζ�HA��Һʱ����Һ��ʱΪ���ԣ����жϴ�ʱ��Һ�����Ϊ������NaOH��NaA��d�����HCl�ζ�BOH��Һʱ����Һ��ʱΪ���ԣ����ж���Һ�����ΪBCl������HCl������c��d��ʱ�μ�NaOH����HCl������ͬ������c��d��ʱ��ƽ��ʱNaOH��NaA��BCl��HCl�����ϱ���ͬ����Ϊ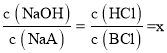 �����ݷ�Ӧ��ϵ�ͳ�ʼ������ϵ����֪c��d����Һ���֮������Ӧ��������Һ����ֹ�ϵΪx��c(NaCl)��1��c(NaA)��1��c(BCl)�����ݵ���غ㣬c(Na+)+c(H+)+c(B+)=c(OH)+c(A)+c(Cl)�����������غ㣬c(A)=c(B+)+c(BOH)��c(Na+)=c(Cl)���ۺϿ��ǣ�����c (H+)�Tc(OH)+c(BOH)����D��ȷ��
�����ݷ�Ӧ��ϵ�ͳ�ʼ������ϵ����֪c��d����Һ���֮������Ӧ��������Һ����ֹ�ϵΪx��c(NaCl)��1��c(NaA)��1��c(BCl)�����ݵ���غ㣬c(Na+)+c(H+)+c(B+)=c(OH)+c(A)+c(Cl)�����������غ㣬c(A)=c(B+)+c(BOH)��c(Na+)=c(Cl)���ۺϿ��ǣ�����c (H+)�Tc(OH)+c(BOH)����D��ȷ��
�ʴ�ѡ��D��


 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҿ���NaBr��ŨH2SO4���Ҵ�Ϊԭ���Ʊ����������飺
C2H5��OH+HBr![]() C2H5Br+H2O
C2H5Br+H2O
��֪��Ӧ�������Ϊ��0.30 mol NaBr��s����0.25 mol C2H5OH���ܶ�Ϊ0.80 g��cm-3����36 mLŨH2SO4����������Ϊ98%���ܶ�Ϊ1.84 g��mL-1����25 mLˮ���Իش��������⡣
��1����ʵ��Ӧѡ��ͼ�е�aװ�û���bװ�ã�_____________��
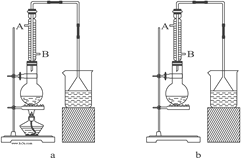
��2����Ӧװ���е���ƿӦѡ���������ֹ���������_____��
A.50 mL B.100 mL C.150 mL D.250 mL
��3���������е�����ˮ������Ӧ����_____��
A. A��B�� B. B��A�� C. ��A����B������
��4�����ܷ����ĸ���ӦΪ��_____________��__________��______________������д��3������ʽ����
��5��ʵ����ɺ��뽫��ƿ�ڵ��л�������������õ��ػ�ɫ�Ĵ������飬���ô��������飬Ӧ���õĴ�ʩ��_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(N2H4)�Ͱ���Ϊ��Ҫ�Ļ���ԭ�ϡ��ش��������⣺
��֪��I.N2H4(l)+O2(g)![]() N2(g)+2H2O(l) ��H=-624.0 kJ/mol
N2(g)+2H2O(l) ��H=-624.0 kJ/mol
II.N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4 kJ/mol
2NH3(g) ��H=-92.4 kJ/mol
III.2NH3(g)![]() N2H4(l)+H2(g) ��H=+144.8 kJ/mol
N2H4(l)+H2(g) ��H=+144.8 kJ/mol
(1)H2��ȼ���ȡ�H=_____________��
(2)T1 ��Cʱ������ݵ��ܱ������м���1 mol N2H4��1 mol O2��������ӦI���ﵽƽ���ֻ�ı�������������ʹN2��ƽ����������������_______( ��ѡ����ĸ)��
A.����ѹǿ B.��ͨ��һ����O2
C.���������ˮ D.�����¶�
(3)�ں�ѹ���ȵ��ܱ�������ͨ��һ������N2��H2��������ӦII�ͷ�ӦIII����ӦIII��N2��ƽ��ת���ʵ�Ӱ��Ϊ_____(��������������С��������Ӱ����)������Ϊ___________��
(4)t2��Cʱ������������г���NH3��������ӦIII��NH3��H2�ķ�ѹ(p)��ʱ��(t)�Ĺ�ϵ��ͼ��ʾ��
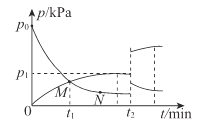
��0~t1min�ڣ���Ӧ��ƽ������v(NH3)=____kPa/min
�ڷ�Ӧ��ƽ�ⳣ��Kp=______kPa-1 (KpΪ�÷�ѹ��ʾ��ƽ�ⳣ��)��
�۷�Ӧ����ӵ���Ч��ײ���ʣ�M____N(����>����<������=��)��
��t2 minʱ�����¶ȣ��ٴδﵽƽ���H2�ķ�ѹ�����ԭ��Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��WΪ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Yԭ�ӵ������������ڲ��������3��������˵����ȷ���� (����)
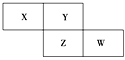
A. ԭ�Ӱ뾶��W>Z>XB. �ǽ����ԣ�Z>Y
C. ����ϼۣ�X>ZD. ����������Ӧˮ��������ԣ�W>Z
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������ܹ��ɹ����ǣ� ��
A.���� Na2O2�����Ƿ����Ϊ Na2CO3���������м������ᣬ������ɫ��ζ������
B.����ij±�����Ƿ����ȴ����� ���� ![]() ��ȴ
��ȴ![]()
![]() ���ְ�ɫ����
���ְ�ɫ����
C.��ȥ�������л��е��壺���Һ![]()
![]() ��Һ�ֲ�
��Һ�ֲ�![]() ���²�
���²�
D.������ A �ijɷ��� FeBr2��
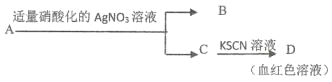
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£��ֱ�ȡδ֪Ũ�ȵ�MOH��HA��Һ����ˮϡ����ԭ�����n����ϡ�����У�����ҺpH�ı仯����ͼ��ʾ������������ȷ����

A. MOHΪ���HAΪǿ��
B. ˮ�ĵ���̶ȣ�X=Z>Y
C. �������¶ȣ�Y��Z���Ӧ��Һ��pH������
D. ��X����Һ��Z����Һ�������ϣ�������Һ�ʼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ף�POCl3���㷺����ũҩ��ҽҩ����������ҵ�Ʊ��������Ĺ����л��������Ʒ�����ᣨH3PO3�����ش��������⣺
��1���������������Ȼ��ס�ˮ���������ȷ�Ӧ���ɣ���Ӧ�Ļ�ѧ����ʽΪ_______
��2����֪�����ᣨH3PO3��Ϊ��Ԫ���ᣬ��Na2HPO3��Һ�У�������Ũ�ȵĴ�С��ϵΪ_______
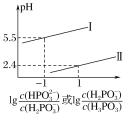
��3�������£���NaOH��Һ�μӵ������ᣨH3PO3����Һ�У������Һ��pH������Ũ�ȱ仯�Ĺ�ϵ��ͼ��ʾ�����ʾlg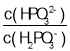 ��������_____���������������������������ᣨH3PO3����Ka1��_____����ӦHPO32-��H2O
��������_____���������������������������ᣨH3PO3����Ka1��_____����ӦHPO32-��H2O![]() H2PO3-��OH����ƽ�ⳣ����ֵ��_____��
H2PO3-��OH����ƽ�ⳣ����ֵ��_____��
��4����ҵ��������������ͬʱ���������ˮ����Ҫ�ɷ�ΪH3PO4��H3PO3�������ˮ���ȼ�������Ư�ۣ��ټ�����ʯ�ҵ���pH������Ԫ��ת��Ϊ����ĸ��γ��������ա���������ķ�ˮ��c��Ca2������5��10��6 mol��L��1������Һ��c��PO43-����_____ mol��L��1������֪Ksp[Ca3(PO4)2]��2��10��29��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.�����������ڹ�ũҵ�����ж�����ҪӦ�á�
(1)������(N2H4)����������ĵ��⻯�
��֪��4NH3(g)��3O2(g)2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g)N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ___________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=__________(��K1��K2��ʾ)��
(2)����2NO(g)��2CO(g)N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
��������˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����____________(����ĸ����)��
A.c(CO)=c(CO2) B.�����л��������ܶȲ���
C.v(N2)��=2v(NO)�� D.�����л�������ƽ��Ħ����������
��ͼΪ�����ڵ�ѹǿ(P)����ʼѹǿ(P0)�ı�ֵ(P/P0)��ʱ��(t)�ı仯���ߡ�0��5min�ڣ��÷�Ӧ��ƽ����Ӧ����v(N2)= _____________��ƽ��ʱNO��ת����Ϊ______________��(��֪�����ѹǿ�ȵ��������ʵ���֮��)
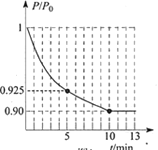
(3)ʹ�ü�ӵ绯ѧ���ɴ���ȼú�����е�NO��װ����ͼ��ʾ����֪���ص�����������Һ��pH��4��7֮�䣬д�������ĵ缫��Ӧʽ________________��
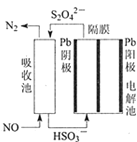
��.���ð�ˮ���������е� SO2
����ˮ�� SO2ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��_________��(��ᡱ�)�� ������������ʵĵ���ƽ�ⳣ�����£���ˮ��Kb=1.8��10-5molL��1��H2SO3 �� Ka1=1.3��10-2molL��1��Ka2=6.3��10-8molL��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��һ�������£���ͼ��ʾװ�ÿ�ʵ���л���ĵ绯ѧ���⣨���������л��������˵��������ǣ�������
��֪������Ч�ʣ�����![]() ��100%��
��100%��
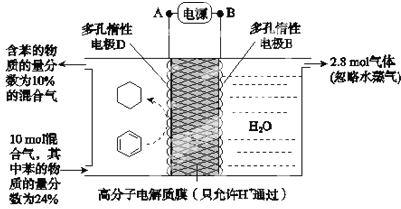
A.��ԴBΪ����
B.�����е����ƶ�����ΪA��D
C.�缫D��ӦʽΪC6H6+6H++6e-=C6H12
D.�ô���װ�õĵ���Ч������24.3%
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com