
��7�֣���֪20��ʱNaCl���ܽ��Ϊ36 g��ij�ȼʹ�õ��豸�������ӽ���Ĥ���ۣ���һ��������м���һ����20��ʱ����NaCl��Һ����90%��NaCl���ʱ�������ռ���11. 2 m3���壨������ɱ�״��������ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2��ȡ��������Һ��ϵ��ʵ�飬���н����д������ ��������ĸ��
| A���μӷ�̪�Լ����ȱ�����ɫ | B���μ���������Һ���а�ɫ�������� |
| C���μ�С�մ���Һ�������ݲ��� | D������ɫ��Ӧʵ��ʻ�ɫ |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������� | ʵ������ | ʵ��Ŀ�� |
| ����II�����������ܽ�õ���Һ�� |
||
| ����III�� |
||
| ����IV�����ˣ�����Һ�� |
||
| ����V������Һ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������ѧ2011��2012ѧ��߶���ѧ�ڵ������¿���ѧ���� ���ͣ�022
��֪20��ʱNaCl���ܽ��Ϊ36 g��ij�ȼʹ�õ��豸�������ӽ���Ĥ���ۣ���һ��������м���һ����20��ʱ����NaCl��Һ����90����NaCl���ʱ�������ռ���11.2 m3����(������ɱ�״��)����ش��������⣺
(1)�÷�Ӧ�����ӷ���ʽΪ________��
(2)ȡ��������Һ��ϵ��ʵ�飬���н����д������________��(����ĸ)
A���μӷ�̪�Լ����ȱ�����ɫ
B���μ���������Һ���а�ɫ��������
C���μ�С�մ���Һ�������ݲ���
D������ɫ��Ӧʵ��ʻ�ɫ
(3)����ԭ����NaCl��Һ������________kg(��ȷ��0.1 kg)��
(4)�����90����NaCl����������ɼ���ȼ�ϵ���ṩ���������������________m3��
(����ĵ缫��Ӧʽ��CH4��10OH�D�D8e����CO32����7H2O
�����ȼ�ϵ�ص�����������Ϊ90��������ɱ�״������ȷ��0.1 m3)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�ع��и�����һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
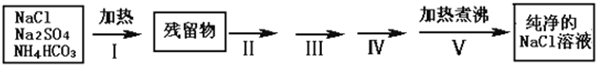
ʵ������Ҫ������NaCl��Һ����ʵ���ҵ�NaCl�����������Na2SO4��NH4HCO3��ijͬѧ����������ͼ���ʵ���ȥ���ʣ��ش��������⣺

��1������I��ȥ�������ǣ��ѧʽ��_______________��ֱ�Ӽ���Ҫ���ڼ�ǿ����ٽ��м��ȣ������� ��
��2��������ͼ���ʵ����ƣ�����ص�ʵ�������ʵ�������ʵ��Ŀ����д���±��У�
|
�������� |
ʵ������ |
ʵ��Ŀ�� |
|
����II�����������ܽ�õ���Һ�� |
|
|
|
����III��
|
|
|
|
����IV�����ˣ�����Һ��
|
|
|
|
����V������Һ������� |
|
|
��3�������õ�20���NaCl������Һ����֪20��ʱNaCl���ܽ��Ϊ36.0g��NaCl������Һ���ܶ�Ϊ1.12g/cm3 ����20���NaCl������Һ�����ʵ���Ũ��Ϊ mol/L��������������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ������
��7�֣���֪20��ʱNaCl���ܽ��Ϊ36 g��ij�ȼʹ�õ��豸�������ӽ���Ĥ���ۣ���һ��������м���һ����20��ʱ����NaCl��Һ����90%��NaCl���ʱ�������ռ���11. 2 m3���壨������ɱ�״��������ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2��ȡ��������Һ��ϵ��ʵ�飬���н����д������ ��������ĸ��
A���μӷ�̪�Լ����ȱ�����ɫ B���μ���������Һ���а�ɫ��������
C���μ�С�մ���Һ�������ݲ��� D������ɫ��Ӧʵ��ʻ�ɫ
��3������ԭ����NaCl��Һ������ kg����ȷ��0.1kg����
��4�������90%��NaCl����������ɼ���ȼ�ϵ���ṩ��������������� m3��
������ĵ缫��Ӧʽ��CH4+10OH��-8e�� = CO32��+7H2O
�����ȼ�ϵ�ص�����������Ϊ90%������ɱ�״������ȷ��0.1 m3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com