
����Ŀ����1��______molH2O�к��е���ԭ������1.5molCO2�к��е���ԭ������ȡ�
��2���������ʵ�����NH3��CH4��ϣ����������NH3��CH4��������Ϊ________��
��3��ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���NH3��CH4�����ʵ���֮��Ϊ__________��
��4����״���£��ܶ�Ϊ0.75g��L��1��NH3��CH4��ɵĻ�������У�NH3���������Ϊ__________���û�����������������ܶ�Ϊ________��
��5����֪agA��bgBǡ����ȫ��Ӧ����0.2molC��dgD����C��Ħ������Ϊ__________��
���𰸡� 3 17��16 4��3 80% 8.4 5(a+b-d)g/mol
��������(1)������ԭ����Ŀ��ȣ���ˮ�����ʵ���Ϊ![]() =3mol���ʴ�Ϊ��3��
=3mol���ʴ�Ϊ��3��
(2)����m=nM��֪�������ʵ�����NH3��CH4��������Ϊ17g/mol��16g/mol=17��16���ʴ�Ϊ��17��16��
(3)ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���![]() =
=![]() �����
�����![]() =
=![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� ![]() ��
��
(4)�������ƽ��Ħ������Ϊ0.75gL-1��22.4L/mol=16.8g/mol����NH3���������Ϊx����17x+16(1-x)=16.8�����x=0.8=80%���û�����������������ܶ�Ϊ![]() =8.4���ʴ�Ϊ��80%��8.4��
=8.4���ʴ�Ϊ��80%��8.4��
(5)���������غ㶨�ɣ�C������Ϊ(a+b-d)g����C��Ħ������Ϊ![]() =5(a+b-d)g/mol���ʴ�Ϊ��5(a+b-d)g/mol��
=5(a+b-d)g/mol���ʴ�Ϊ��5(a+b-d)g/mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��������Cl2й©������Ӧ�Դ�ʩ����Ҫ�ǻ���Cl2���������ʿ��ǵ���
A. ����Ⱦ��������ʯ��
B. ��Զ����ȾԴ�ĸߴ�����
C. �ý��д�����Һ��ë����ס�ڱ�Ѹ�ٳ���
D. ����������ʱ���ô�����Һ��ʪ���ޱ��º��Ŵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʵ����Ǹ��л�ѧ���õ�������������������йؼ��㣺
(1)0.2 g H2����________��Hԭ�ӡ�
(2)��״���£�������ͬ��ԭ������CO��CO2�����֮��Ϊ________��
(3)100 mL��������Һ��n(Al3��)��0.20 mol(������ˮ������)��������c(SO![]() )��________��
)��________��
(4)��9.5 gij���۽������Ȼ����к���0.2 mol Cl�������Ȼ����Ħ������Ϊ________���ý���Ԫ�ص����ԭ������Ϊ________��
(5)6.72 L CO(��״��)��һ������Fe2O3ǡ����ȫ��Ӧ(����Fe��CO2)������Fe������Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��������ӵ�ص��ܷ�ӦʽΪ2Li+FeS=Fe+Li2S��LiPF6��SO(CH3)2Ϊ����ʣ��øõ��Ϊ��Դ��⺬�����Է�ˮ���õ�����Ni��ʵ��װ����ͼ��ʾ������˵������ȷ����

A. �缫YΪLi
B. �������У�b��NaCl��Һ�����ʵ���Ũ�Ƚ����ϼ�С
C. X����ӦʽΪFeS+2Li++2e-=Fe+Li2S
D. ����ͼ��������Ĥȥ������a��b���Һϲ������ⷴӦ�ܷ���ʽ�����ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ͼ����ʵ�飬��A�зŵ��Ǹ���ĺ�ɫֽ����B�зŵ���ʪ��ĺ�ɫֽ����C��ʢ�ŵ�������������Һ����ش��������⡣
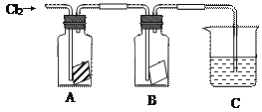
��1��ͨ��Cl2һ��ʱ���A��Bװ���У���ɫֽ��������ͽ���Ϊ��
װ�� | ���� | ���� |
A | ______________ | ��ʪ������______�����ܻ��ܣ�ʹ��ɫֽ����ɫ����Ϊ��Ӧ���ɵ�____________����Ư���� |
B | ______________ |
��2��Cװ�õ�������__________________________
д��װ��C�з�Ӧ�Ļ�ѧ����ʽ��_________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ƫ������[(NaPO3)6]��ƫ������(NaPO3)��һ�־ۺ��壬����Ҫ����ˮ��������ֽ��ʳƷ��ҵ����ҵ���ɰ��ף�P4������ȼ���۵�44�棬�е�280�棬�о綾����������ˮ�У��Ʊ���ƫ�����Ƶķ������£�
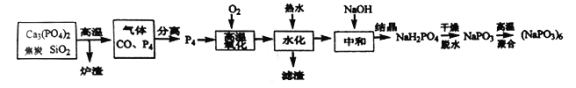
��1����¯��������Ҫ�ɷ���___________(д��ѧʽ)���ù������������뻹ԭ�������ʵ���֮����_______________��
��2���ӻ�������з���õ�P4����ѷ�����_________________��
��3�������������������ɰ����е�Pb��As����Ԫ�����γɵ��������࣬����һ����Pb3(PO4)2������һ����_______________ (д��ѧʽ)��
��4����ֱ�ӽ�����������ŨNaOH��Һֱ�ӻ�ϼ��ȣ���õ����Ǵ�������(NaH2PO2)����ͬʱ��õ�һ�־綾���塣д���û�ѧ����ʽ____________________��
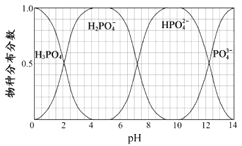
��5����֪�����к������ֵķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���������ҺpH�Ĺ�ϵ��ͼ��ʾ�����к͡�������Ӧ����NaOH������Һ��pHֵΪ________________����pH���ͣ�����ɵĽ����_________________��
��6����������������100.00g��Ʒ����Ч�ף���P2O5�ƣ�������66.50g (��������Ϊ�������γɷ�)����ò�Ʒ�Ĵ�����__________________��(��֪��NaPO3����Է�������ΪM1��P2O5����Է�������ΪM2��������ػ����г�����ʽ���ɣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������NH4Cl��MgSO4��(NH4)2SO4 ��NaCl������ɫ��Һ����һ���Լ��Ϳɽ����Ǽ�������������Լ���
A. NaOH B. Ba(OH)2 C. AgNO3 D. HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ���ǣ� ��
A.��H��0�ķ�Ӧ�����Է���Ӧ
B.�Է����еķ�Ӧһ����Ѹ�ٽ���
C.�����������Զ��ۻ���ˮ���������ӵĽ��
D.������ؼ��ȷֽ���һ���ؼ�С�Ĺ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Һ�У���������һ���ܹ�����������ǣ�( )
A��ʹ��̪��Һ������Һ�� Na+��Cl-��SO42-��Fe3+
B��ʹ��ɫʯ����Һ������Һ��Fe2+��Mg2+��NO3-��Cl-
C��c(H+)=10-12 mol��L-1����Һ��K+��Ba2+��Cl-��Br-
D��̼��������Һ��K+��SO42-��Cl-��H+
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com