
����ͼ��ʾ��װ���У�A����������װ�ã�C��D Ϊ���徻��װ�ã�C��װ�б���ʳ��ˮ��D ��װ��Ũ���ᣩ��E ��Ӳ�ʲ�����װ��ϸ��˿����FΪ����Ŀչ��ƿ���ձ�G ��װ������������Һ��
�Իش�
��1��ʵ�����������Ļ�ѧ����ʽ��_______________________________��
��2��Cװ�õ�������________________��D װ�õ�������__________________��
E�з�����ѧ��Ӧ�ķ���ʽΪ��_____________________________��
��3���ձ�G ��װ������������Һ��������___________________��������Ӧ�Ļ�ѧ����ʽΪ��_______________________________________��
 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����һ�и߶���һ����ѡ4����ѧ�Ծ����������� ���ͣ�ʵ����
100ml0.50mol��L-1������100mL0.55mol��L-1NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȡ��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ���������
��2���ձ���������ֽ���������� ��
��3��ʵ����������110mL0.50mol��L-1�����100mL0.55mol��L-1NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� �����ȡ�����ȡ����������к��� �����ȡ�����ȡ�����
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ�� �����ƫ��ƫС������Ӱ�족����
��5�������ȼ��н�22.3�桢100ml 1.0mol/L���������������¶ȵ�1.00mol/L������������Һ��ϣ��¶�������ߵ�29.00�档��֪���ȼƵ�������46.1J/K���������µ�NaCl��Һ�ı�����Ϊ4.03J/��g.K������Һ���ܶ�Ϊ1.02g/ml���Լ�������ÿĦNaCl��
��Ӧ�ȣ�ֱ��д�𰸣� KJ/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ͭ�����и߶��ῼ��ϰ��ѧ�Ծ������� ���ͣ�ʵ����
����ͼ��ʾ��װ���У�A����������װ�ã�C��D Ϊ���徻��װ�ã�C��װ�б���ʳ��ˮ��D ��װ��Ũ���ᣩ��E ��Ӳ�ʲ�����װ��ϸ��˿����FΪ����Ŀչ��ƿ���ձ�G ��װ������������Һ��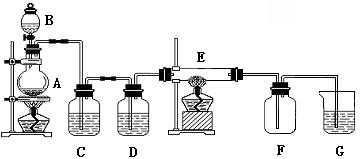
�Իش�
��1��ʵ�����������Ļ�ѧ����ʽ��_______________________________��
��2��Cװ�õ�������________________��D װ�õ�������__________________��
E�з�����ѧ��Ӧ�ķ���ʽΪ��_____________________________��
��3���ձ�G ��װ������������Һ��������___________________��������Ӧ�Ļ�ѧ����ʽΪ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ�����и�һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
50ml0.50mol��L-1������50mL0.55mol��L-1NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȡ�

�ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�������
�� ��װ���л����ڵ�2�������Ǣ�__
�� ���ִ�����������¶ȶ���________�����������С������Ӱ�족������õ��к��Ƚ�____________���ƫ����ƫС������Ӱ�족����
��2����ʵ������У���ͬѧ��Ҫ�ⶨ����¼��ʵ���������� ������ţ���
A�������Ũ��
B��������¶�
C������������Һ��Ũ��
D������������Һ���¶�
E��ˮ�ı�����
F����Ӧ������Һ����ֹ�¶�
��3��ʵ���и���60mL0��50mol��L-1�����50mL0��55mol��L-1NaOH��Һ���з�Ӧ����������ȷ��ʵ�������ȣ����ų������� �����ȡ�����ȡ����������к��� �����ȡ�����ȡ�����
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ�� �����ƫ��ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��16�֣���1�����к͵ζ����������У������¸�����������������ʵ�����á�ƫ�ߡ�����ƫ�͡�����Ӱ�족��գ�
�ٵζ���������ˮϴ����δ����֪Ũ�ȵı���Һ��ϴ��ʹ�ζ���� ��
����ƿ������ˮϴ�������ô�����Һ��ϴ��ʹ�ζ���� ��
�۵ζ���(װ����Һ)�ڵζ�ǰ���촦�����ݣ��ζ����������ݣ�ʹ�ζ���� ��
�ܵζ�ǰƽ�ӣ��ζ����˸��ӣ�ʹ�ζ���� ��
���ú�Na2O���ʵ�NaOH������������֪Ũ�ȵı���Һ�����ڵζ�δ֪Ũ�ȵ����ᣬʹ��������Ũ�� ��
��ϴ����ƿʱ�����ϡʳ��ˮ��������ˮ��Ȼ������ƿװ��������ᣬ��NaOH����Һ�ζ�ʱ���Բ�õĽ�� ��
��2����֪H+(aq)+OH-(aq) = H2O(l) ��H= ��57.3kJ��mol��1����50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

�ٴ�ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ��������� ��
�ڴ��ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ����ƫС������Ӱ�족)��
����ͨ��ʵ��ⶨ�к��ȵĦ�H�������������ڣ�57.3kJ��mol��1����ԭ������ǣ�
��
��3���ֱ���ƻ�ѧʵ�飬����ѷ���֤����������ˮʱ���������б仯(��ѡ�õ�ҩƷ��������������Һ��ʯ����Һ����̪��Һ��pH��ֽ���ƾ���)��
��֤������������ˮ�ⷴӦ ��
��֤����ˮ�ⷴӦ��һ�����ȷ�Ӧ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com