
ЁОЬтФПЁПвбжЊЯТСаШШЛЏбЇЗНГЬЪНЃК
ЂйH2(g)ЃЋ1/2O2(g)ЃНH2O(l)ЁЁІЄHЃНЃ285.8 kJЁЄmolЃ1
ЂкH2(g)ЃЋ1/2O2(g)ЃНH2O(g)ІЄHЃНЃ241.8 kJЁЄmolЃ1
ЂлC(s)ЃЋ1/2O2(g)ЃНCO(g)ЁЁІЄHЃНЃ110.5 kJЁЄmolЃ1
ЂмC(s)ЃЋO2(g)ЃНCO2(g)ЁЁІЄHЃНЃ393.5 kJЁЄmolЃ1ЃЌ
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЩЯЪіЗДгІжаЪєгкЗХШШЗДгІЕФЪЧ____________ЁЃ
ЃЈ2ЃЉH2ЕФШМЩеШШЮЊ____________ kJЁЄmolЃ1ЃЛCЕФШМЩеШШЮЊ____________ kJЁЄmolЃ1ЁЃ
ЃЈ3ЃЉШМЩе10 g H2ЩњГЩвКЬЌЫЎЃЌЗХГіЕФШШСПЮЊ____________ kJЁЃ
ЃЈ4ЃЉаДГіCOШМЩеЕФШШЛЏбЇЗНГЬЪН__________________________________ЁЃ
ЁОД№АИЁПЂйЂкЂлЂм 285.8 kJ /mol 393.5kJ /mol 1429 CO(g) +1/2O2 (g) ===CO2(g) ЁїH=ЁЊ283kJ /mol
ЁОНтЮіЁП
(1)ЮяжЪЕФШМЩеЮЊЗХШШЗДгІЃЌЗХШШЗДгІЕФІЄHЃМ0ЃЛ(2)ШМЩеШШЪЧжИдк101kPЪБЃЌ1molПЩШМЮяЭъШЋШМЩеЩњГЩЮШЖЈЕФбѕЛЏЮяЪБЫљЗХГіЕФШШСПЃЌCдЊЫизЊЛЏЮЊCO2ЃЌHдЊЫизЊЛЏЮЊвКЬЌЫЎЃЌОнДЫЗжЮіХаЖЯЃЛ(3)ЧѓГіЧтЦјЕФЮяжЪЕФСПЃЌШЛКѓИљОн1molЧтЦјШМЩеЩњГЩвКЬЌЫЎЪБЗХГі285.8kJЕФШШСПРДМЦЫуЃЛ(4)ИљОнИЧЫЙЖЈТЩПЩжЊЃЌНЋЂм-ЂлПЩЕУCOЕФШМЩеЗДгІЃЛОнДЫЗжЮіНтД№ЁЃ
(1)ЮяжЪЕФШМЩеОљЮЊЗХШШЗДгІЃЌЗХШШЗДгІЕФІЄHЃМ0ЃЌЖјЂйЂкЮЊЧтЦјЕФШМЩеЃЌЂлЂмЮЊCЕФШМЩеЃЌЙЪЂйЂкЂлЂмОљЮЊЗХШШЗДгІЃЌЙЪД№АИЮЊЃКЂйЂкЂлЂмЃЛ
(2)ШМЩеШШЪЧжИдк101kPЪБЃЌ1molПЩШМЮяЭъШЋШМЩеЩњГЩЮШЖЈЕФбѕЛЏЮяЪБЫљЗХГіЕФШШСПЃЌCдЊЫизЊЛЏЮЊCO2ЃЌHдЊЫизЊЛЏЮЊвКЬЌЫЎЃЌЙЪЗДгІЂйЮЊЧтЦјЕФШМЩеШШЕФШШЛЏбЇЗНГЬЪНЃЌМДЧтЦјЕФШМЩеШШЮЊ285.8kJmol-1ЃЛЗДгІЂмЮЊCЕФШМЩеШШЕФШШЛЏбЇЗНГЬЪНЃЌЙЪCЕФШМЩеШШЮЊ393.5kJmol-1ЃЌЙЪД№АИЮЊЃК285.8kJmol-1ЃЛ393.5kJmol-1ЃЛ
(3)10gЧтЦјЕФЮяжЪЕФСПЮЊ5molЃЌЖј1molЧтЦјШМЩеЩњГЩвКЬЌЫЎЪБЗХГі285.8,kJЃЌЙЪ5molЧтЦјШМЩеЩњГЩвКЬЌЫЎЪБЗХГіЕФШШСПЮЊ285.8kJmol-1ЁС5mol=1429.0kJЃЌЙЪД№АИЮЊЃК1429.0ЃЛ
(4)ИљОнИЧЫЙЖЈТЩПЩжЊЃЌНЋЂм-ЂлПЩЕУCOЕФШМЩеЗДгІЮЊЃКCO(g)+![]() O2(g)ЈTCO2(g) ЁїH=-283.0kJmol-1ЃЌЙЪД№АИЮЊЃКCO(g)+
O2(g)ЈTCO2(g) ЁїH=-283.0kJmol-1ЃЌЙЪД№АИЮЊЃКCO(g)+![]() O2(g)ЈTCO2(g) ЁїH=-283.0kJmol-1ЁЃ
O2(g)ЈTCO2(g) ЁїH=-283.0kJmol-1ЁЃ


 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЁЂEЪЧКЫЕчКЩЪ§вРДЮдіДѓЕФЮхжжЖЬжмЦкжїзхдЊЫиЃЌAдЊЫиЕФдзгКЫФкжЛга1ИіжЪзгЃЛBдЊЫиЕФдзгАыОЖЪЧЦфЫљдкжїзхжазюаЁЕФЃЌBЕФзюИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЕФЛЏбЇЪНЮЊHBO3ЃЛCдЊЫидзгЕФзюЭтВуЕчзгЪ§БШДЮЭтВуЖр4ЃЛCЕФвѕРызггыDЕФбєРызгОпгаЯрЭЌЕФЕчзгХХВМЃЌСНдЊЫиПЩаЮГЩЛЏКЯЮяD2CЃЛCЁЂEЭЌжїзхЁЃ
(1)BдкжмЦкБэжаЕФЮЛжУЮЊЕк________жмЦкЕк________зхЁЃ
(2)EдЊЫиаЮГЩЕФбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЕФЛЏбЇЪНЮЊ____________________ЁЃ
(3)дЊЫиCЁЂDЁЂEаЮГЩЕФМђЕЅРызгАыОЖДѓаЁЙиЯЕЪЧ ______>______>_____(гУРызгЗћКХБэЪО)ЁЃ
(4)гУЕчзгЪНБэЪОЛЏКЯЮяD2CЕФаЮГЩЙ§ГЬЃК____________________ЁЃCЁЂDЛЙПЩаЮГЩЛЏКЯЮяD2C2ЃЌD2C2жаКЌгаЕФЛЏбЇМќЪЧ________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПСзЛЏЧт(PH3)ЪЧвЛжжОчЖОЦјЬхЃЌЪЧзюГЃгУЕФИпаЇбЌеєЩБГцМСЃЌвВЪЧвЛжжЕчзгЙЄвЕдСЯЁЃ
(1)PH3ЕФЕчзгЪНЮЊ______________ЁЃ
(2)дкУмБеСИВжЗХжУЕФСзЛЏТС(AlP)ЦЌМСЃЌгіЫЎеєЦјЗХГіPH3ЦјЬхЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК_____ЁЃ
(3)PH3ЕФвЛжжЙЄвЕжЦЗЈЩцМАЕФЮяжЪзЊЛЏЙиЯЕШчЯТЭМЫљЪОЃК

ЂйДЮСзЫсЪєгк____дЊЫсЃЌбЧСзЫсЪєгк____дЊЫсЃЛ
ЂкЕБЗДгІIЩњГЩЕФn(NaH2PO2):n(Na2HPO3) =3:1ЪБЃЌВЮМгЗДгІЕФn(P4)ЃКn(NaOH)= ____ЁЃ
(4)вЛжжгУгкДІРэPH3ЗЯЦјЕФЮќЪеМСГЩЗжЮЊДЮТШЫсИЦ80%ЁЂОтФОаМ(ЪшЫЩМС)15%ЁЂЛюадЬП 2.5%ЁЂЛЌЪЏЗл(ЗРНсПщ)2.5%ЁЃ
ЂйДЮТШЫсИЦНЋPH3бѕЛЏЮЊH3PO4ЕФЛЏбЇЗНГЬЪНЮЊ_______________________________ЃЛ
ЂкПеЦјжаЕФЫЎеєЦјПЩМгПьPH3ЕФбѕЛЏЙ§ГЬЃЌЦфдвђПЩФмЪЧ_______________________ЁЃ
(5)Дг(4)жаЕФЮќЪеВаСєЮяжаЛиЪеСзЫсЧтИЦ(CaHPO4)ЕФЗНЗЈШчЯТЃК

ЂйЪдМСxЮЊ_________ЬюЛЏбЇЪН)ЃЛ
ЂквбжЊ25ЁцЪБЃЌH3 PO4ЕФKal=7.5ЁС10-3ЁЂKa2=6.3ЁС10-8ЁЂKa3 =4.4ЁС10-13ЁЃМгШыYЪБЃЌгІПижЦаќзЧвКpH____7(ЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБ)ЃЌЭЈЙ§МЦЫуЫЕУїРэгЩЃК________________________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈЛђБэЪОЗНЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A. ЕШжЪСПЕФСђеєЦјКЭСђЙЬЬхЗжБ№дкбѕЦјжаЭъШЋШМЩеЃЌКѓепЗХГіЕФШШСПЖр
B. гЩC(ЪЏФЋ)=C(Н№ИеЪЏ) ІЄH= +11.9 kJ/molЃЌПЩжЊН№ИеЪЏБШЪЏФЋЮШЖЈ
C. ЫЎСІЃЈЫЎФмЃЉАДВЛЭЌЕФЗжРрПЩПДГЩПЩдйЩњФмдДКЭвЛМЖФмдД
D. БэЪОЧтЦјШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊH2(g)+1/2O2(g)=H2O(g) ІЄH= -241.8 kJ/mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЕчРыЗНГЬЪНжаЃЌе§ШЗЕФЪЧ
A. CH3COOH = CH3COO- + H+ B. NaOH = Na+ + OH-
C. KClO3![]() K+ + ClO3- D. BaSO4 = Ba2+ + S2- +4O2-
K+ + ClO3- D. BaSO4 = Ba2+ + S2- +4O2-
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП25 ЁцЪБЃЌВПЗжЮяжЪЕФЕчРыГЃЪ§ШчБэЫљЪОЃК
ЛЏбЇЪН | CH3COOH | H2CO3 | HClO |
ЕчРыГЃЪ§ | 1.7ЁС10Ѓ5 | K1ЃН4.3ЁС10Ѓ7ЃЌK2ЃН5.6ЁС10Ѓ11 | 3.0ЁС10Ѓ8 |
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉCH3COOHЁЂH2CO3ЁЂHClOЕФЫсадгЩЧПЕНШѕЕФЫГађЮЊ______________ЁЃ
ЃЈ2ЃЉЭЌХЈЖШЕФCH3COOЃЁЂHCO3-ЁЂCO32-ЁЂClOЃНсКЯHЃЋЕФФмСІгЩЧПЕНШѕЕФЫГађЮЊ______ЁЃ
ЃЈ3ЃЉГЃЮТЯТ0.1 molЁЄLЃ1ЕФCH3COOHШмвКдкМгЫЎЯЁЪЭЙ§ГЬжаЃЌЯТСаБэДяЪНЕФЪ§ОнвЛЖЈБфаЁЕФЪЧ___________(ЬюађКХ)ЃЌвЛЖЈВЛБфЕФЪЧ________(ЬюађКХ)ЁЃвЛЖЈБфДѓЕФЪЧ________(ЬюађКХ)ЃЌ
AЃЎc(HЃЋ) BЃЎc(HЃЋ)/c(CH3COOH) CЃЎc(HЃЋ)ЁЄc(OHЃ)
DЃЎc(OHЃ)/c(HЃЋ) E. c(CH3COOЃ)ЁЄc(HЃЋ)/c(CH3COOH)
ШєИУШмвКЩ§ИпЮТЖШЃЌЩЯЪі5жжБэДяЪНЕФЪ§ОндіДѓЕФЪЧ____________(ЬюађКХ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПWЁЂXЁЂYЁЂZЮЊдзгађЪ§вРДЮдіДѓЕФЧАЫФжмЦкдЊЫиЃЌдЊЫиWЪЧгюжцжазюЗсИЛЕФдЊЫиЃЌдЊЫиXЕФдзгзюЭтВуЕчзгЪ§ЪЧЦфФкВуЕФ3БЖЃЌдЊЫиZЕФЛљЬЌдзгКЫЭтЕчзгга24жждЫЖЏзДЬЌЃЌYЁЂXЁЂZВЛдкЭЌвЛжмЦкЃЌЧвYдзгКЫЭтpЕчзгБШsЕчзгЖр5ИіЁЃ
(1)ZЛљЬЌдзгЕФКЫЭтЕчзгХХВМЪНЮЊ__________ЁЃ
(2)ZЕФбѕЛЏЮяЪЧЪЏгЭЛЏЙЄжаживЊЕФДпЛЏМСжЎвЛЃЌШчДпЛЏвьБћБН(![]() )СбЛЏЩњГЩБНКЭБћЯЉЁЃ
)СбЛЏЩњГЩБНКЭБћЯЉЁЃ
Ђй1molБћЯЉЗжзгжаКЌгаІвМќгыІаМќЪ§ФПжЎБШЮЊ_______ЁЃ
ЂкБНЗжзгжаЬМдзгЙьЕРЕФдгЛЏРраЭЮЊ__________ЁЃ
ЂлZЕФвЛжжбѕЛЏЮяZO5жаЃЌZЕФЛЏКЯМлЮЊ+6ЃЌдђЦфжаЙ§бѕМќЕФЪ§ФПЮЊ_______ИіЁЃ
(3)WЁЂXЁЂYШ§жждЊЫиЕФЕчИКадгЩаЁЕНДѓЫГађЮЊ__________ЁЃ(ЧыгУдЊЫиЗћКХЛиД№)
(4)ZY3ШлЕуЮЊ1152ЁцЃЌШлШкзДЬЌЯТФмЙЛЕМЕчЃЌОнДЫПЩХаЖЯZY3ОЇЬхЪєгк__________(ЬюОЇЬхРраЭ)ЁЃ
(5)ZX2ОЇЬхЕФОЇАћНсЙЙШчЭМЃЌУПИіZдзгжмЮЇзюНќЕФX дзгЪ§ФПЮЊ__________ЁЃШєИУЛЏКЯЮяЕФЯрЖдЗжзгжЪСПЮЊMЃЌОЇАћБпГЄЮЊacmЃЌАЂЗќМгЕТТоГЃЪ§ЮЊNAЃЌдђИУОЇЬхЕФУмЖШЮЊ_________g/cm3ЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПбЮЫсЦеТГПЈвђ ( 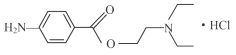 ) ЪЧвЛжжСМКУЕФОжВПТщзэвЉЃЌ ОпгаЖОадаЁЃЌ ЮоГЩёЋадЕШЬиЕуЁЃЦфКЯГЩТЗЯпШчЯТЭМЫљЪОЃК
) ЪЧвЛжжСМКУЕФОжВПТщзэвЉЃЌ ОпгаЖОадаЁЃЌ ЮоГЩёЋадЕШЬиЕуЁЃЦфКЯГЩТЗЯпШчЯТЭМЫљЪОЃК

ЛиД№ЯТСаЮЪЬтЃК
(1) 3molAПЩвдКЯГЩ1molBЃЌ ЧвBЪЧЦНУце§СљБпаЮНсЙЙЃЌ дђBЕФНсЙЙМђЪНЮЊ_________ЁЃ
(2) гаЛњЮяCЕФУћГЦЮЊ____________ЃЌ BЁњCЕФЗДгІРраЭЮЊ_____________ЁЃ
(3) ЗДгІCЁњDЕФЛЏбЇЗНГЬЪНЮЊ________________________________________ЁЃ
(4) FКЭEЗЂЩњѕЅЛЏЗДгІЩњГЩGЃЌ дђFЕФНсЙЙМђЪНЮЊ___________________ЁЃ
(5) HЕФЗжзгЪНЮЊ____ЁЃ
(6) ЗжзгЪНЮЊC9H12ЧвЪЧCЕФЭЌЯЕЮяЕФЭЌЗжвьЙЙЬхЙВга__________жжЁЃ
(7) ЧыНсКЯЩЯЪіСїГЬаХЯЂЃЌ ЩшМЦгЩБНЁЂ ввШВЮЊдСЯКЯГЩ ЕФТЗЯп_______________ ЁЃ(ЦфЫћЮоЛњЪдМСШЮбЁ)
ЕФТЗЯп_______________ ЁЃ(ЦфЫћЮоЛњЪдМСШЮбЁ)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПРћгУЯТСаЪЕбщзАжУФмДяЕНЪЕбщФПЕФЕФЪЧ
A.  ЗжРыCH3COOHКЭCH3COOC2H5ЛьКЯвК
ЗжРыCH3COOHКЭCH3COOC2H5ЛьКЯвК
B.  бщжЄNH4NO3ОЇЬхШмгкЫЎЕФШШаЇгІ
бщжЄNH4NO3ОЇЬхШмгкЫЎЕФШШаЇгІ
C.  еєЗЂFeCl3ШмвКЕУЕНFeCl3ЙЬЬх
еєЗЂFeCl3ШмвКЕУЕНFeCl3ЙЬЬх
D. 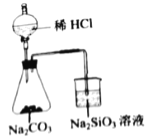 бщжЄCЁЂClЁЂSiЕФЗЧН№ЪєадЧПШѕ
бщжЄCЁЂClЁЂSiЕФЗЧН№ЪєадЧПШѕ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com