
���ӹ�ҵ����30%��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��,����ӡˢ��·�塣ij����ʦΪ�˴ӷ�Һ�л���ͭ,���»��FeCl3��Һ,���������ʵ�鲽��: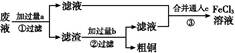
д��һ����֤����ԭ��Fe��Cuǿ�����ӷ���ʽ: ��
�÷�Ӧ����ͼ���� �з����������������Ӧ���һ��ԭ���,�ڷ����л�������װ��ͼ(����缫���ơ��缫���ϡ��������Һ)��
| |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ƽ�IJ�������ͼ��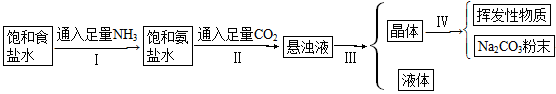
�Իش��������⣺
(1)������������Һ��NaHCO3��NH4Cl�Ļ��Һ�����͢���ܷ�ӦΪ
_______________________________________________
(2)����һ��Ӧ��֪NaCl��NaHCO3�ܽ��______________________��
(3)��IJ�����____________��
(4)���IJ�����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ����ٺ����ʡ�ݣ��ܹ�������ú�ˮ�Ƶö��ֻ�����Ʒ����ͼ���Ժ�ˮ�����ǣ���Ҫ�ɷ�CaCO3����Ϊԭ����ȡ���ֻ�����Ʒ�Ĺ�������ͼ������E��һ�ֻ��ʣ�N��һ�ֳ����Ľ������ʡ�
��������������̻ش��������⣺
��1������G��L�Ļ�ѧʽ�ֱ�Ϊ �� ������B�������е�һ�ֱ���Ʒ����Ҫ����
��2���������������п���ѭ��ʹ�õ����ʵĻ�ѧʽΪ
��3����Ӧ�ٵĻ�ѧ����ʽΪ ���ڷ�Ӧ���б�����ͨ��NH3������ͨ��D����ԭ����
��4����ҵ������F���Ƶ���һ�ֻ�����Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ
��5����K��Һ����δ��������Ƶ�N��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C���������о�����ͬһ��Ԫ�أ�����֮��������ͼ��ʾ��ת����ϵ(���ַ�Ӧ������ȥ)��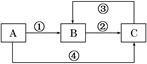
��1����A��һ�����������B��ˮ��Һ�����ԣ���д����Ӧ�ٺͷ�Ӧ��(��Aһ��ת��ΪB��C)�����ӷ���ʽ����Ӧ��______________________________________��
��Ӧ��_______________________________________________________��
��2������Ӧ��Ϊ�û���Ӧ����Ӧ��Ϊ���Ϸ�Ӧ��C���ʴ��������ں�ˮ�У����������������ȱ�ٵ����ʡ���ҵ�Ͽ�����C����ȡA��B����д����������Ӧ�Ļ�ѧ����ʽ��C��A________________________________________________________________________��
C��B_______________________________________________________________��
��3������Ӧ�٢ڢ۾�Ϊ���Ϸ�Ӧ����Ӧ��Ϊ�û���Ӧ����AΪһ�ֽ�������ʱ����д����Ӧ�ں͢ۿ��ܵ����ӷ���ʽ����_____________________________________________��
��_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���㷺Ӧ���ڻ�ѧ��ҵ���ճ������С���ҵ����������(Al2O3��3H2O�ĺ���ԼΪ85%,������ҪΪSiO2��Fe2O3��)ұ�����������������¡�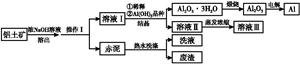
��֪�ݶ�������Al2O3��3H2O�Ļ���ԭ��Ϊ:
Al2O3��3H2O+2NaOH(aq) 2NaAlO2(aq)+4H2O,[Al2O3��3H2OҲ�ɱ�ʾΪ2Al(OH)3]
2NaAlO2(aq)+4H2O,[Al2O3��3H2OҲ�ɱ�ʾΪ2Al(OH)3]
(1)�����������Ϊ��������,�����г���������������,�����е���Ҫ������ ��
(2)Ϊ�������������ܳ����ʿɲ�ȡ����Ч��ʩΪ (��д����)��
(3)�û�ѧƽ�����۽���ϡ����Һ��������Al2O3��3H2O �ᾧ��ԭ���� ��
(4)Ϊ������Al2O3��3H2O,Ҳ������Һ����ͨ�����CO2����,д������Al2O3��3H2O�����ӷ���ʽ: ����
(5)Ϊ�˻��ճ����Һ���ߵ����óɷ�,��ҵ�Ͻ�����ˮϴ�Ӻ��ϴҺ������Һ���ϡ�ͼ�,��ָ������ͼ����һ�����Ƶ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����������Ϊԭ���Ʊ��������ѵĹ�����������ͼ��ʾ�����������Ҫ�ɷ�Ϊ��������(FeTiO3)������һ������Ԫ���ڷ绯�����л�ת��Ϊ��3�ۡ�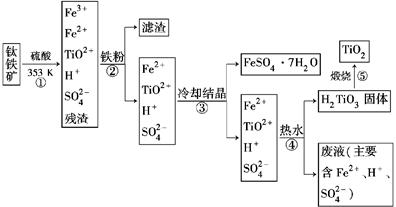
��֪��TiOSO4��ˮ��ˮ�⡣
(1)������У������۽�Fe3��ת��ΪFe2�������ӷ���ʽΪ_______________________
(2)������У�ʵ�ֻ����ķ������������ʵ�________(����ĸ���)��
a���ۡ��е���졡 b���ܽ��Բ��졡 c�������ԡ���ԭ�Բ���
(3)����ڡ��ۡ����У�����Ҫ���еIJ�����________(���������)��
(4)���ϻ�ѧ�����û�ѧƽ�����۽��Ͳ�����н�TiO2��ת��ΪH2TiO3��ԭ����
____________________________________________________________��
(5)�������������еķ�Һ�����̿�(��Ҫ�ɷ�ΪMnO2)��Ӧ������������(MnSO4��������ˮ)���÷�Ӧ�����ӷ���ʽΪ__________________________________
(6)�о����֣���ʯī������������������������CaF2��CaO������ʣ�������ͼ��ʾװ�ÿɻ�ý����ƣ������Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
д�������ĵ缫��Ӧʽ��_________________________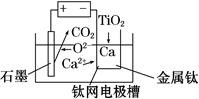
���Ʊ�������ǰ��CaO���������䣬��ԭ����______________________________________(���ϻ�ѧ�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��G����ͼ��ʾ��ת����ϵ����������������ȥ��������A��GΪ���ʣ�D����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬E��F������NaOH��Һ��Ӧ��
��ش��������⣺
��1��д��F�ĵ���ʽ��____________��
��2����C��Һ��D��Ӧ�����ӷ���ʽΪ________________________________________________________________________________________________________________________________________________��
��F��Һ��NaOH��Һ���ȷ�Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________________________________________________________________________________��
��3�����������ӷ���ʽ����C��ҺΪ�������ԣ�________________________________________________________________________________________________________________________________________________��
��F��Һ������Ũ���ɴ�С��˳��Ϊ________________________________________________________________________��
��4����5.4 g AͶ��200 mL 2.0 mol/Lij��Һ����G���ʲ������ҳ�ַ�Ӧ���н���ʣ�࣬�����Һ������________������ţ���
A��HNO3��Һ B��H2SO4��Һ C��NaOH��Һ�� D��HCl��Һ
��5����1 mol N2��3 mol G�����������ݻ�Ϊ2 L��ij�ܱ������н��з�Ӧ����֪�÷�ӦΪ���ȷ�Ӧ��ƽ��ʱ�����D�����ʵ���Ũ��Ϊa mol/L��
�������Ӧ����v��G����1.2 mol/��L��min������v��D����________mol/��L��min����
���������������������£�����ʼʱ����0.5 mol N2��1.5 mol G�ﵽƽ���D�����ʵ���Ũ��________������ڡ���С�ڡ����ڡ��� mol/L��
mol/L��
�۸������µ�ƽ�ⳣ��Ϊ__________________���ú�a�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����̼�������������ᷴӦ���ж�����̼����ų������������������̼���Ʒ�Ӧ��������̼�����ƺ��Ȼ��ƣ�������ų�������A��B��ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ��ƿ��Һ�ijɷּ����ʵ���Ũ�ȣ���������ʵ�飺
��ȡ20mLA��Һ�������л�������B��Һ25mL�����ռ���112mL����״�������塣
��ȡ25mLB��Һ�������л�������A��Һ20mL�����ռ���56mL����״�������塣
��1��д���������������̼���Ʒ�Ӧ��������ų������ӷ���ʽ ��
��2��Ϊʹ�����٢ڷ�Ӧ��ȫ��������� �����ϡ���ᡱ��̼������Һ����A��Һ�����ʵ���Ũ��Ϊ mol��L -1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���廯����A������ͼ��ʾ����ϵ�б仯����֪E��Һ�м��백ˮ������İ�ɫ�����ܿ��Ϊ����ɫ������Ϊ���ɫ��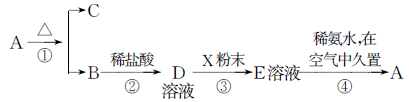
��1��д���������ʵĻ�ѧʽ��
A��__________��B��__________��D��__________��E��__________��X��__________��
��2��д����Ӧ�ٵĻ�ѧ����ʽ��________________________________��
д����Ӧ�۵����ӷ���ʽ�� ________________________________��
д����Ӧ�ܹ����У���ɫ�����ڿ����о��õĻ�ѧ����ʽ��________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com