
ЁОЬтФПЁПH ЪЧКЯГЩПЙбзвЉТхнгТхЗвФЦЕФЙиМќжаМфЬхЃЌЫќЕФвЛжжКЯГЩТЗЯпШчЯТЃК

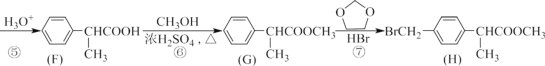
(1)A ЕФЮяжЪУћГЦЮЊ_________ЃЌH жаЗЧКЌбѕЙйФмЭХУћГЦЪЧ_________ЁЃ
(2)E ЕФНсЙЙМђЪНЮЊ_________ЃЌЗДгІЂоЕФЗДгІРраЭЮЊ_________ЁЃ
(3)гІЂйЕФЛЏбЇЗНГЬЪНЮЊ_________ЃЌЗДгІЂоЕФЛЏбЇЗНГЬЪНЮЊ_________ЁЃ
(4)аДГіТњзуЯТСаЬѕМўЕФ F ЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪН_________ЁЃ
I.ФмЗЂЩњЫЎНтЗДгІЩњГЩЫсКЭДМ
Ђђ.ФмЗЂЩњвјОЕЗДгІ
Ђѓ.КЫДХЙВеёЧтЦзга 5 зщЗхЧвЗхУцЛ§жЎБШЮЊ 3:2:2:2:1
(5)ЗТееH ЕФКЯГЩЯпЃЌЩшМЦвЛжжгЩ![]() КЯГЩ
КЯГЩ![]() ЕФКЯГЩТЗЯп________ЁЃ
ЕФКЯГЩТЗЯп________ЁЃ
ЁОД№АИЁПМзБН фхдзг  ѕЅЛЏЗДгІ(ЛђШЁДњЗДгІ)
ѕЅЛЏЗДгІ(ЛђШЁДњЗДгІ) ![]()
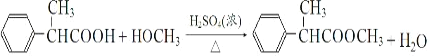
![]()
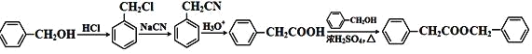
ЁОНтЮіЁП
гЩAЁЂBЁЂCЕФЗжзгЪНЃЌНсКЯDЕФНсЙЙМђЪНЃЌИљОнзЊЛЏЙиЯЕПЩжЊЃЌAЮЊ![]() ЃЌBЮЊ
ЃЌBЮЊ![]() ЃЌCЮЊ
ЃЌCЮЊ![]() ЁЃЖдБШDЁЂFНсЙЙПЩжЊDЗЂЩњШЁДњЗДгІЩњГЩEЮЊ
ЁЃЖдБШDЁЂFНсЙЙПЩжЊDЗЂЩњШЁДњЗДгІЩњГЩEЮЊ ЃЌFгыМзДМЗЂЩњѕЅЛЏЗДгІЩњГЩGЃЌGЗЂЩњШЁДњЗДгІЕУЕНHЃЌЭЌЪБЛЙЩњГЩввЖўДМЃЛБНМзДМгыHClЗЂЩњШЁДњЗДгІЩњГЩ
ЃЌFгыМзДМЗЂЩњѕЅЛЏЗДгІЩњГЩGЃЌGЗЂЩњШЁДњЗДгІЕУЕНHЃЌЭЌЪБЛЙЩњГЩввЖўДМЃЛБНМзДМгыHClЗЂЩњШЁДњЗДгІЩњГЩ![]() ЃЌдйгыNaCNЗЂЩњШЁДњЗДгІЕУЕН
ЃЌдйгыNaCNЗЂЩњШЁДњЗДгІЕУЕН![]() ЃЌЫсадЬѕМўЯТЫЎНтЕУЕН
ЃЌЫсадЬѕМўЯТЫЎНтЕУЕН![]() ЃЌзюКѓгыБНМзДМЗЂЩњѕЅЛЏЗДгІЕУЕН
ЃЌзюКѓгыБНМзДМЗЂЩњѕЅЛЏЗДгІЕУЕН![]() ЁЃ
ЁЃ
(1)AЮЊ![]() ЃЌAЕФУћГЦЪЧМзБНЃЛHжаКЌгаЕФЗЧКЌбѕЙйФмЭХУћГЦЪЧЃКѕЅЛљЁЂфхдзгЃЌЙЪД№АИЮЊЃКМзБНЃЛфхдзгЁЃ
ЃЌAЕФУћГЦЪЧМзБНЃЛHжаКЌгаЕФЗЧКЌбѕЙйФмЭХУћГЦЪЧЃКѕЅЛљЁЂфхдзгЃЌЙЪД№АИЮЊЃКМзБНЃЛфхдзгЁЃ
(2)ИљОнЧАУцЗжЮіЕУЕНEЕФНсЙЙМђЪНЮЊ ЃЌЗДгІЂоЪЧѕЅЛЏЗДгІ(ЛђШЁДњЗДгІ)ЃЛЙЪД№АИЮЊЃК
ЃЌЗДгІЂоЪЧѕЅЛЏЗДгІ(ЛђШЁДњЗДгІ)ЃЛЙЪД№АИЮЊЃК ЃЛѕЅЛЏЗДгІ(ЛђШЁДњЗДгІ)ЁЃ
ЃЛѕЅЛЏЗДгІ(ЛђШЁДњЗДгІ)ЁЃ
(3)ЗДгІЂйЕФЛЏбЇЗНГЬЪНЮЊЃК![]() ЃЌЗДгІЂоЕФЛЏбЇЗНГЬЪНЮЊЃК
ЃЌЗДгІЂоЕФЛЏбЇЗНГЬЪНЮЊЃК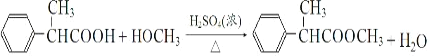 ЃЛЙЪД№АИЮЊЃК
ЃЛЙЪД№АИЮЊЃК![]() ЃЛ
ЃЛ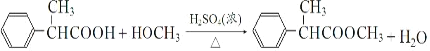 ЁЃ
ЁЃ
(4)ТњзуЯТСаЬѕМўЕФFЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃКI.ФмЗЂЩњЫЎНтЗДгІЩњГЩЫсКЭДМЃЌКЌгаєШЫсгыДМаЮГЩЕФѕЅЛљЃЛЂђ.ФмЗЂЩњвјОЕЗДгІЃЌКЌгаМзЫсгыДМаЮГЩЕФѕЅЛљЃЌЂѓ.КЫДХЙВеёЧтЦзга5зщЗхЧвЗхУцЛ§жЎБШЮЊ3:2:2:2:1ЃЌЗћКЯЬѕМўЕФЭЌЗжвьЙЙЬхНсЙЙМђЪНЮЊЃК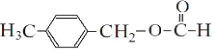 ЃЛЙЪД№АИЮЊЃК
ЃЛЙЪД№АИЮЊЃК![]() ЁЃ
ЁЃ
(5)БНМзДМгыHClЗЂЩњШЁДњЗДгІЩњГЩ![]() ЃЌдйгыNaCNЗЂЩњШЁДњЗДгІЕУЕН
ЃЌдйгыNaCNЗЂЩњШЁДњЗДгІЕУЕН![]() ЃЌЫсадЬѕМўЯТЫЎНтЕУЕН
ЃЌЫсадЬѕМўЯТЫЎНтЕУЕН![]() ЃЌзюКѓгыБНМзДМЗЂЩњѕЅЛЏЗДгІЕУЕН
ЃЌзюКѓгыБНМзДМЗЂЩњѕЅЛЏЗДгІЕУЕН![]() ЃЌКЯГЩТЗЯпСїГЬЭМЮЊЃК
ЃЌКЯГЩТЗЯпСїГЬЭМЮЊЃК ЃЛЙЪД№АИЮЊЃК
ЃЛЙЪД№АИЮЊЃК ЁЃ
ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдЊЫиXЮЛгкЕк4жмЦкЃЌЦфЛљЬЌдзгЕФФкВуЙьЕРШЋВПХХТњЕчзгЃЌЧвзюЭтВуЕчзгЪ§ЮЊ2ЃЛдЊЫиYЛљЬЌдзгЕФ3pЙьЕРЩЯга1ЖдГЩЖдЕчзгЁЃXгыYаЮГЩЕФЛЏКЯЮяЕФОЇАћНсЙЙШчЭМЫљЪОЃЌЯТСаЙигкИУОЇЬхЕФЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
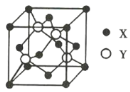
A.ИУОЇЬхЪєгкдзгОЇЬх
B.X2+ЕФХфЮЛЪ§ЮЊ8ЃЌY2-ЕФХфЮЛЪ§ЮЊ4
C.гыУПИіY2-ОрРызюНќЧвЯрЕШЕФY2-ЙВга12Иі
D.ИУОЇЬхЕФШлЕуБШбѕЛЏаПИп
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЧПЫсадЮоЩЋШмвКжаПЩФмКЌЯТБэРызгжаЕФШєИЩжжРызгЁЃ
бєРызг | Mg2+ЁЂNH4+ЁЂBa2+ЁЂAl3+ЁЂFe2+ |
вѕРызг | SiO32-ЁЂMnO4-ЁЂCl-ЁЂNO3-ЁЂSO42- |
ЪЕбщЂё:ШЁЩйСПИУЧПЫсадШмвКAНјааШчЯТЪЕбщЁЃ
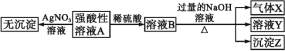
ЪЕбщЂђ:ЮЊСЫНјвЛВНШЗЖЈИУШмвКЕФзщГЩ,ШЁ100 mLдШмвКA,ЯђИУШмвКжаЕЮМг1 molЁЄL-1ЕФNaOHШмвК,ВњЩњГСЕэЕФжЪСПгыЧтбѕЛЏФЦШмвКЬхЛ§ЕФЙиЯЕШчЭМЫљЪОЁЃ
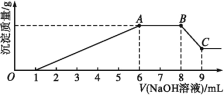
ЛиД№ЯТСаЮЪЬт:
(1)ВЛНјааЪЕбщОЭПЩвдЭЦЖЯГі,ЩЯБэжаЕФРызгвЛЖЈВЛДцдкЕФга________жжЁЃ
(2)ЭЈЙ§ЪЕбщЂёПЩвдШЗЖЈИУШмвКжавЛЖЈДцдкЕФвѕРызгЪЧ_________ЁЃМьбщЦјЬхXЕФЗНЗЈЪЧ_______________;ГСЕэZЕФЛЏбЇЪНЮЊ_____________ЁЃ
(3)аДГіЪЕбщЂђЕФЭМЪОжаBCЖЮЖдгІЗДгІЕФРызгЗНГЬЪН:________________ЁЃ
(4)AЕуЖдгІЕФЙЬЬхжЪСПЮЊ____ gЁЃ
(5)ЭЈЙ§ЩЯЪіаХЯЂ,ЭЦЫуИУШмвКжавѕРызгЕФХЈЖШЮЊ________ molЁЄL-1ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЮТЖШЪБЃЌVIAдЊЫиЕЅжЪгыH2ЗДгІЩњГЩЦјЬЌH2XЕФШШЛЏбЇЗНГЬЪНШчЯТЃК
![]() O2(g) +H2(g) ЃНH2O(g)
O2(g) +H2(g) ЃНH2O(g) ![]() HЃНЃ242kJЁЄmolЃ1
HЃНЃ242kJЁЄmolЃ1
S(g)+ H2(g) ЃНH2S(g) ![]() HЃНЃ20kJЁЄmolЃ1
HЃНЃ20kJЁЄmolЃ1
Se(g)+H2(g)![]() H2Se(g)
H2Se(g) ![]() HЃН+81kJЁЄmolЃ1
HЃН+81kJЁЄmolЃ1
ЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. ЮШЖЈадЃКH2O< H2S< H2Se
B. НЕЮТгаРћгкSeгыH2ЗДгІЩњГЩH2Se
C. O2(g)+2H2S(g)ЃН2H2O(g)+2S(g) ![]() HЃНЃ444 kJЁЄmolЃ1
HЃНЃ444 kJЁЄmolЃ1
D. ЫцзХКЫЕчКЩЪ§ЕФдіМгЃЌVIAзхдЊЫиЕЅжЪгыH2ЕФЛЏКЯЗДгІдНШнвзЗЂЩњ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAЁЂBЁЂCЁЂDОљЮЊжабЇЛЏбЇГЃМћЕФДПОЛЮяЃЌAЪЧЕЅжЪЁЃЫќУЧжЎМфгаШчЯТЕФЗДгІЙиЯЕЃК
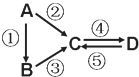
(1)ШєDЮяжЪОпгаСНадЃЌЂкЂлЗДгІОљвЊгУЧПМюШмвКЃЌЂмЗДгІЪЧЭЈШыЙ§СПЕФвЛжжв§Ц№ЮТЪваЇгІЕФжївЊЦјЬхЁЃаДГіЂкЗДгІЕФРызгЗНГЬЪН_______________________________________ЃЛЂмЗДгІРызгЗНГЬЪН__________________________________________________ЁЃ
(2)ШєAЪЧгІгУзюЙуЗКЕФН№ЪєЁЃЂмЗДгІгУЕНAЃЌЂкЂнЗДгІОљгУЕНЭЌвЛжжЛЦТЬЩЋЦјЬхЕЅжЪЁЃаДГіAгыЫЎИпЮТЯТЗДгІЕФЛЏбЇЗНГЬЪН_________________________________ЁЃЂмЗДгІЕФРызгЗНГЬЪН_________________________ЁЃDжаМгШыЧтбѕЛЏФЦЕФЯжЯѓ_____________________ЁЃ
(3)ШєAЪЧЬЋбєФмЕчГигУЕФЙтЗќВФСЯЃЌBГЃгУгкжЦзїИпЕЕЙтбЇЦїВФЃЌCЁЂDЮЊФЦбЮЃЌCЕФЫЎШмвКЫзГЦЫЎВЃСЇЃЌDЕФШмвКЯдМюадЁЃЂмЗДгІвВЪЧЭЈШывЛжжв§Ц№ЮТЪваЇгІЕФжївЊЦјЬхЁЃаДГіЂлЗДгІЕФЛЏбЇЗНГЬЪН___________________________________ЁЃЂнЗДгІгУЕНBЃЌЗДгІЬѕМўЮЊИпЮТЃЌдђЂнЕФЛЏбЇЗНГЬЪНЮЊ___________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАБЕФДпЛЏбѕЛЏЪЧЙЄвЕжЦЯѕЫсЕФживЊЗДгІЃК4NH3ЃЋ5O2![]() 4NOЃЋ6H2OЃЌЖдгкИУЗДгІХаЖЯе§ШЗЕФЪЧ
4NOЃЋ6H2OЃЌЖдгкИУЗДгІХаЖЯе§ШЗЕФЪЧ
A. бѕЦјБЛЛЙдB. ИУЗДгІЪЧжУЛЛЗДгІ
C. АБЦјЪЧбѕЛЏМСD. Шєга17 gАБВЮМгЗДгІЃЌЗДгІжазЊвЦ10 molЕчзг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
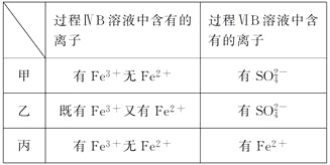
ЁОЬтФПЁПЮЊбщжЄбѕЛЏадЃКCl2ЃОFe3ЃЋЃОSO2ЃЌФГаЁзщгУЯТЭМЫљЪОзАжУНјааЪЕбщ(МаГжвЧЦїКЭAжаЕФМгШШзАжУвбТдЃЌЦјУмадвбОМьбщЭъБЯ)ЪЕбщЙ§ГЬШчЭМЃК
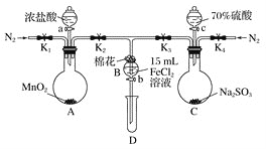
Ђё.ДђПЊЕЏЛЩМаK1ЁЋK4ЃЌЭЈШывЛЖЮЪБМфN2ЃЌдйНЋTаЮЕМЙмВхШыBжаЃЌМЬајЭЈШыN2ЃЌШЛКѓЙиБеK1ЁЂK3ЁЂK4ЁЃ
Ђђ.ДђПЊЛюШћaЃЌЕЮМгвЛЖЈСПЕФХЈбЮЫсЃЌИјAМгШШЁЃ
Ђѓ.ЕБBжаШмвКБфЛЦЪБЃЌЭЃжЙМгШШЃЌМаНєЕЏЛЩМаK2ЁЃ
Ђє.ДђПЊЛюШћbЃЌЪЙдМ2 mLЕФШмвКСїШыDЪдЙмжаЃЌМьбщЦфжаЕФбєРызгЁЃ
Ђѕ.ДђПЊЕЏЛЩМаK3ЁЂЛюШћcЃЌМгШы70%ЕФСђЫсЃЌвЛЖЮЪБМфКѓМаНєЕЏЛЩМаK3ЁЃ
Ђі.ИќаТЪдЙмDЃЌжиИДЙ§ГЬЂєЃЌМьбщBШмвКжаЕФРызгЁЃ
(1)Й§ГЬЂёЕФФПЕФЪЧ________________________ЁЃ
(2)УоЛЈжаНўШѓЕФШмвКЮЊ__________________ЁЃзїгУЪЧ_______________________ЁЃ
(3)AжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЃК______________________________________ЁЃ
(4)ЕМжТВНжшЂѓжаШмвКБфЛЦЕФРызгЗДгІЪЧ______________________________ЁЃгУ______________(аДЪдМСЛЏбЇЪН)МьбщбѕЛЏВњЮяЃЌЯжЯѓЪЧ___________________________ЁЃ
(5)ФмЫЕУїбѕЛЏадFe3ЃЋЃОSO2ЕФРызгЗНГЬЪНЪЧ__________________________ЁЃ
(6)МзЁЂввЁЂБћШ§ЮЛЭЌбЇЗжБ№ЭъГЩСЫЩЯЪіЪЕбщЃЌЫћУЧЕФМьВтНсЙћвЛЖЈФмЙЛжЄУїбѕЛЏадCl2ЃОFe3ЃЋЃОSO2ЕФЪЧ________________(ЬюЁАМзЁБЁАввЁБЛђЁАБћЁБ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЪЧжабЇЛЏбЇжаГЃМћЮяжЪжЎМфЕФвЛаЉЗДгІЙиЯЕЃЌЦфжаВПЗжВњЮяЮДаДГіЁЃГЃЮТЯТXЪЧЙЬЬхЃЌBКЭGЪЧвКЬхЃЌЦфгрОљЮЊЦјЬхЁЃИљОнЯТЭМЙиЯЕЭЦЖЯЃК

(1)аДГіЛЏбЇЪНЃКX_______ЃЌA________ЃЌB_______ЁЃ
(2)ЪЕбщЪвЪеМЏЦјЬхDКЭFЕФЗНЗЈвРДЮЪЧ_______ЗЈЁЂ________ЗЈЁЃ
(3)аДГіCЁњEЕФЛЏбЇЗНГЬЪНЃК____________ЁЃ
(4)ЧыаДГіAгыEЗДгІЩњГЩDЕФЛЏбЇЗНГЬЪНЃК_______________
(5)МьбщЮяжЪAЕФЗНЗЈКЭЯжЯѓЪЧ________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУЯТУцЕФдзгЛђРызгНсЙЙЪОвтЭМЕФБрКХ(AЁЂBЁЂCЁЂD)ЬюПеЃК
![]()
(1)ЕчзгВуХХВМЯрЭЌЕФЪЧ______________ЃЛ
(2)ЪєгкЭЌжждЊЫиЕФЪЧ________________ЃЛЁЁЁЁЁЁЁЁ
(3)ЪєгкН№ЪєдЊЫиЕФЪЧ ___________________ЃЛЁЁ
(4)ЪєгкЯЁгаЦјЬхдЊЫиЕФЪЧ______________ЃЌЯЁгаЦјЬхвЛАуВЛВЮМгЛЏбЇЗДгІЕФдвђЪЧ_______
(5)аДГіDВЮМгЛЏбЇЗДгІКѓЫљЕУРызгЕФНсЙЙЪОвтЭМ _____________
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com