
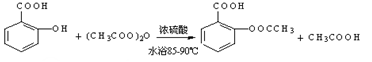
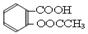 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128~135�档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128~135�档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�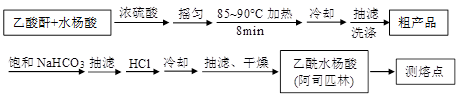
| �� �� | ��Է������� | �۵��е㣨�棩 | ˮ |
| ˮ���� | 138 | 158(�۵�) | �� |
| ������ | 102 | 139.4(�е�) | ��Ӧ |
| ����ˮ���� | 180 | 135(�۵�) | �� |
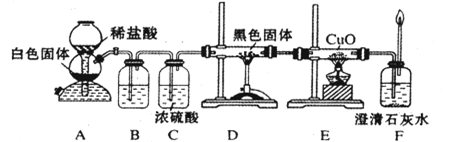
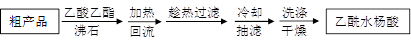 �Ľ����ᴿ�����м��Ȼ�����װ����ͼ��ʾ
�Ľ����ᴿ�����м��Ȼ�����װ����ͼ��ʾ

 ��n(������)=
��n(������)=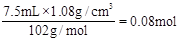 ��������������������ˮ����0.02mol�������Ϊ
��������������������ˮ����0.02mol�������Ϊ ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 KMnO4 + H2C2O4 + �� ��---K2SO4 + CO2��+ MnSO4 + �� ��
KMnO4 + H2C2O4 + �� ��---K2SO4 + CO2��+ MnSO4 + �� ���鿴�𰸺ͽ���>>
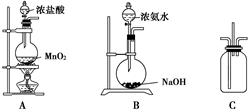
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
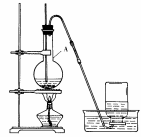
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ۢܢݢޢߢ� | B���ڢۢݢޢߢ�� |
| C���٢ڢۢݢޢߢ� | D���٢ڢۢܢݢޢ� |
�鿴�𰸺ͽ���>>
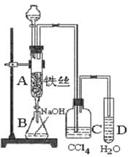
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 CO+H2 CO + H2O
CO+H2 CO + H2O CO2 + H2 C + CO2
CO2 + H2 C + CO2 2CO
2CO 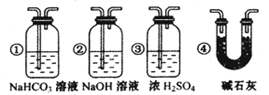
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com