
����Ŀ�����仯����Ӧ�ù㷺���ش��������⣺
��1����̬Bԭ�ӵļ۵��ӹ������ʽΪ________�����һ�����ܱ�Be ________�����С������
��2�������飨NH3BH3������Ϊ�����DZ�������ʹ������֮һ�������д�����λ�����ṩ�µ��ӶԵijɼ�ԭ����________��д��һ���백���黥Ϊ�ȵ�����ķ���________���ѧʽ����

��3�����³�ѹ�����ᣨH3BO3������ṹΪ��״�����άƽ��ṹ��ͼa��
�� Bԭ�ӵ��ӻ���ʽΪ________��������ĽǶȽ�����������ˮ�е��ܽ��С������ʱ�ܽ������________��
�� ·��˹���������Ϊ���κοɽ��ܵ��ӶԵķ��ӻ����ӽ�·��˹�ᣬ�κοɸ������ӶԵķ��ӻ����ӽ�·��˹��ӽṹ�Ƕȷ���������·��˹�________��
��4������������BN�����������ĥ���������ϣ��侧���ṹ����ʯ���ƣ���ͼb��ʾ��
�� �뵪ԭ��ֱ�����ӵ���ԭ�ӹ��ɵļ�����״Ϊ________����ԭ�Ӻ͵�ԭ�������ӵ���С��Ϊ________Ԫ����
�� ��������������Ҫ�أ�
ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�á���ͼb��ʾ������ԭ���������XΪ��0��0��0����Yԭ�ӵ��������Ϊ��1/2��0��1/2������Zԭ�ӵ��������Ϊ________��
�������������������Ĵ�С����״����֪������������ܶ�Ϊd gcm��3�������ӵ�����ֵΪNA��������a��________nm�����г�����ʽ������
���𰸡� 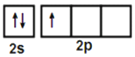 С N C2H6 sp2 ������Ӽ�ͨ������ϣ�����ʱ����������ƻ��� ���������Bԭ����һ��2p�չ���� �������� �� (
С N C2H6 sp2 ������Ӽ�ͨ������ϣ�����ʱ����������ƻ��� ���������Bԭ����һ��2p�չ���� �������� �� (![]() ��
�� ![]() ��
�� ![]() )
) 
����������1��Bԭ��ԭ�Ӻ���5�����ӣ���̬Bԭ�ӵ����Ų�ʽ�ǣ�1s22s22p1�����Ի�̬Bԭ�ӵļ۵��ӹ������ʽΪ��![]() ��Bԭ�Ӽ۵����Ų�Ϊ2s22p1��Beԭ�Ӽ۵����Ų�Ϊ2s2����Ȼp�ܼ������ϸ���������"���ع���"���ȼ۹��ȫ�����߰�������ȫ��������ȶ��Ľṹ����B��һ�����ܱ�BeС��
��Bԭ�Ӽ۵����Ų�Ϊ2s22p1��Beԭ�Ӽ۵����Ų�Ϊ2s2����Ȼp�ܼ������ϸ���������"���ع���"���ȼ۹��ȫ�����߰�������ȫ��������ȶ��Ľṹ����B��һ�����ܱ�BeС��
��2�������飨NH3BH3���У�N��B�Ļ��ϼ۶���-3��BH3��NH3�Ļ��ϼ۶���0������֮����ͨ����λ�������ĵ���B��������������������������Hԭ������ȱ���������Ӿʹ��ȶ��ṹ����N�������H����һ���µ��Ӷԣ�����N�ṩ�µ��ӶԶ�B�ṩ�չ���γ���λ�����ȵ����������ͬ�ĵ�����Ŀ��ԭ����Ŀ��C2H6�백���黥Ϊ�ȵ�������
��3������ԭ�������ֻ��3�����ӣ�����ԭ���γ�3�Թ��õ��Ӷԣ��۲���Ӷ�Ϊ3���ӻ������Ϊ3���ӻ�����Ϊsp2�����ڵ�H3BO3����֮����Ҫͨ���������������ʱ����������ױ��ƻ���������������ˮ�е��ܽ��С������ʱ�ܽ����������������ĵ�Bԭ��sp2�ӻ�����3��sp2��1��2p����������������4��B��3���۵������ֱ���3��sp2����к����ɼ�����ô�ɼ���Ŀ��3����ĵ��Ӷ��õ���������û�й¶Ե�������ô����һ���չ����Ҳ����2P�չ��������·��˹������ۣ��κοɽ��ܵ��ӶԵķ��ӻ����ӽ�·��˹�ᣬ����������·��˹�ᡣ
��4��������������BN����ͼb��N(��ɫ)ռ���������8�������6�����ģ�������8��![]() +6��
+6��![]() =4����B(��ɫ)ռ��С�����壨�˷�֮һ�������������ģ���4������һ����������4��N��4��B���뵪ԭ��ֱ�����ӵ���ԭ�ӹ��ɵļ�����״Ϊ�������壬���е�ԭ�������ģ�4����ԭ���ڶ��㣻��ԭ�Ӻ͵�ԭ�������ӵ���С��Ϊ��Ԫ������ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�á���ͼb��ʾ������ԭ���������XΪ��0��0��0����Yԭ�ӵ��������Ϊ��1/2��0��1/2����ZΪB(��ɫ)ռ��С�����壨�˷�֮һ������������������֪Z���ڵ��������
=4����B(��ɫ)ռ��С�����壨�˷�֮һ�������������ģ���4������һ����������4��N��4��B���뵪ԭ��ֱ�����ӵ���ԭ�ӹ��ɵļ�����״Ϊ�������壬���е�ԭ�������ģ�4����ԭ���ڶ��㣻��ԭ�Ӻ͵�ԭ�������ӵ���С��Ϊ��Ԫ������ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�á���ͼb��ʾ������ԭ���������XΪ��0��0��0����Yԭ�ӵ��������Ϊ��1/2��0��1/2����ZΪB(��ɫ)ռ��С�����壨�˷�֮һ������������������֪Z���ڵ��������![]() ������Zԭ�ӵ��������Ϊ(
������Zԭ�ӵ��������Ϊ(![]() ��
�� ![]() ��
�� ![]() )������֪������������ܶ�Ϊd gcm��3�������ӵ�����ֵΪNA����������Ϊa��N�����ԭ������Ϊ14��B�����ԭ������Ϊ11������ݾ�����������ʽ��d��a3=
)������֪������������ܶ�Ϊd gcm��3�������ӵ�����ֵΪNA����������Ϊa��N�����ԭ������Ϊ14��B�����ԭ������Ϊ11������ݾ�����������ʽ��d��a3=![]() ������a=
������a= cm=
cm=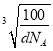 ��107 nm��
��107 nm��


 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)(CH3)2CHCHO����������Ӧ�Ļ�ѧ����ʽ_______________________________________��
(2)������������������µ�ˮ�ⷴӦ�ѷ�����Ӧ��ȡ������Ӧ����ϲ���Һ���Թ��У���__________________________________________________��������_____________________������֤����������ˮ�⡣
(3)��ϵͳ���������� Ϊ____________________��
Ϊ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ��һ����Ҫ���л�����ԭ�ϣ���ṹ��ʽΪ ![]() ����һ�㲻���ܾ��е������� ( D )
����һ�㲻���ܾ��е������� ( D )
A. ������ˮ�����������л��ܼ�
B. �ڿ�����ȼ�ղ�������
C. ����ʹ������Ȼ�̼��Һ��ɫ��Ҳ��ʹ���Ը��������Һ��ɫ
D. �ܷ����ӳɷ�Ӧ����һ�������¿���4�����ʵ����������ӳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������пһͭһϡ������ɵ�ԭ���װ���У�����������1 mol����ͨ��ʱ�������ϵ������仯��
��пƬ�ܽ���32.5g��пƬ���� 32.5g��ͭƬ������lg H2��ͭƬ������1mol H2
A. ������ B. ������ C. ������ D. ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���������NaOH��AlCl3��MgCl2��ɣ���������������ˮ�У���ֽ������0.58g��ɫ�����������õ�����Һ����μ���0.5mol/L�����ᣬ�����������������ɳ�����������g����ϵ��ͼ��ʾ�����ٶ�������NaOH��Һ��Al3+��Mg2+ת��Ϊ�����������Ⱥ�˳��

��1�����������ֵ����m= ___________________g��
��2��������ȫ�ܽ�ʱ����������������a=________________mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Na2O��Na2O2��������ȷ���ǣ� ��
A. ���ǰ�ɫ�Ĺ��� B. ���ܺ�ˮ��Ӧ�γ�ǿ����Һ
C. ���Ǽ��������� D. ��ɫ��Ӧ����ɫ������ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��X��Y��Z��W��Rԭ��������������Xԭ�����������������ڲ��������2����Y��Rͬ���壬�����ߺ��������֮����x�����������4����Z�������ֵķǽ�������ǿ��Ԫ�أ������ڱ���Wλ�ڢ�A�塣
�밴Ҫ��ش�
��1��Ԫ��X�����ڱ�������λ��Ϊ________��
��2��Ԫ��Y��R�ļ��⻯���ȶ�����ǿ������˳��Ϊ________��д�⻯��Ļ�ѧʽ����
��3��Ԫ��Z��W�ļ����Ӱ뾶�ɴ�С��˳������Ϊ________�������ӷ��ţ���
��4��W��R����Ԫ������������Ӧ��ˮ������ˮ��Һ�����Ӧ�����ӷ���ʽΪ____________��
��5����֪���ڼ��������£�R��������W����������ˮ��Һ��Ӧ��������RԪ�صķֱ�Ϊ+4��-2����д���÷�Ӧ�Ļ�ѧ����ʽ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ȷ����
A. ̼������ͬ�������壬������û��
B. ��������ԭ��Ӧ��,�ǽ�������һ����������
C. ijԪ�شӻ���̬��Ϊ����̬ʱ,��Ԫ��һ������ԭ
D. ��������ȡ�ķ������嵥�ʻ�ⵥ�ʴ����ǵ�ˮ��Һ����ȡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���£�N2H4)��һ����ɫ������ˮ����״Һ�塣���м��Ժͼ�ǿ�Ļ�ԭ�ԣ��ڹ�ҵ������Ӧ�÷dz��㷺��

��1����֪�µ����������ͼ1��ʾ����д���µĵ���ʽ��________��
��2��Ŀǰ�����з���һ����ȼ�ϵ�صĽṹ��ͼ2��ʾ��________���a����b�����缫Ϊ��صĸ�����
��3����1L�̶�����������м���0.1molN2H4����303K��Pt���·�����N2H4(I)![]() N2(g)+2H2(g)�����������
N2(g)+2H2(g)�����������![]() ��ʱ���ϵ��ͼ3��ʾ����04min������ƽ������v(N2)=________��
��ʱ���ϵ��ͼ3��ʾ����04min������ƽ������v(N2)=________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com