
�廯��(CaBr2��2H2O)��һ�ְ�ɫ���壬������ˮ���к�ǿ����ʪ�ԣ��ǹ���ֽ��Ȫˮ����������Ҫ�ɷ֣���ҽҩ������������˥���ȵ�ҩ�Ҳ������ѧ�������ù�ҵ����ʯ����������Al3+��Fe3+�����ʣ��Ʊ��廯�Ƶ���Ҫ��������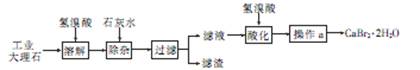
�ش���������
��1���ܽ�ʱ��������Ҫ��Ӧ�����ӷ���ʽΪ
��2�����Ӳ��������Һ��pHԼΪ8��0��Ŀ���� ��
��3����Һ���������ữ��Ŀ���� ������a��Ҫ���� �� ����
��4���Ƶõ��廯�ƾ������ͨ�����²���ⶨ�䴿�ȣ�
�ٳ�ȡ5��00g�廯�ƾ�����Ʒ�����ܽ⣻�۵�������Naa2CO3��Һ����ַ�Ӧ����ˣ��ܺ�ɡ���ȴ���ݳ��������õ�2�� 00 g̼��ƣ�����Ʒ�Ĵ���Ϊ
��5���廯�ƾ����������Ӻ����ӵļ���
�ٽ������廯�ƾ�������ˮ�����������ữ��AgNO3��Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
�ڽ������廯�ƾ�������ˮ���μӲ�������Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
��1��CaCO3��2H��=Ca2����CO2����H2O��2�֣�
��2��ȷ��Al3����Fe3��������ȫ����ֹAl��OH��3�ܽ⣨��1�֣�
��3����ȥ������Ca��OH��2������Ũ������ȴ�ᾧ����1�֣�
��4��94��4%����0��944����2�֣�
��5���ٲ�������ɫ���ǣ�1�֣���Br����Ag��=AgBr����2�֣�
�ڲ�����ɫ������1�֣���Ca2++C2O42-=CaC2O4����2�֣�
�������������������ѧ����������Ҫע�⣺������ͷ����ȷ����������Ŀ�Ģ������̽�������Ϣ��ȷÿһ����ԭ����Ŀ�ĺͲ����۴���Ҫע��淶��ע�⻯ѧ�������дҪ��ʵ�������������������1������ʯ����Ҫ�ɷ�Ϊ̼��ƣ��������ᷴӦ�����廯�ơ�ˮ�Ͷ�����̼�����ӷ���ʽΪCaCO3��2H��=Ca2����CO2����H2O����2��������ҵ����ʯ�ijɷּ�����ͼ֪�����ӵ�Ŀ���dz�ȥAl3����Fe3���������������Ϊ���������������ǿ��ǿ�������Һ��pHԼΪ8��0��Ŀ����ȷ��Al3����Fe3��������ȫ����ֹAl��OH��3�ܽ⣻��3����Һ�к������������ƣ����������ữ��Ŀ���dz�ȥ������Ca(OH)2����Һ�еþ���IJ���Ϊ����Ũ������ȴ�ᾧ�����˵ȣ���4�����������غ㶨��֪��n(CaBr2)= n(CaCO3)=0.02mol���廯�ƾ���(CaBr2��2H2O)������Ϊ4.72g,��������Ϊ4.72/5.00��100%=94��4%����5�����������ữ����������Һ���������ӣ�ʵ������Ϊ��������ɫ���ǣ����ӷ���ʽΪBr����Ag��=AgBr�������ò�������Һ��������ӣ�����Ϊ������ɫ���������ӷ���ʽΪCa2++C2O42-=CaC2O4����
���㣺������ѧ��������Ϊ���忼�����ӷ���ʽ����д����ѧʵ�������������ѧ���㼰���Ӽ��顣


 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����������ᣨ�е㣺90��C��ʱ��ͬʱ�������������ƣ��乤���������£�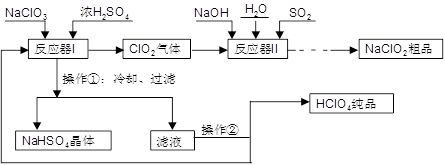
��1��ʵ���ҽ��й��˲����ij��ò��������� ��
��2����Ӧ��I�е��¶����Ϊ ������ţ��������ڵ�����Ϊ ��
A. 0��C �� B. 20��C �� C. 80��C �� D. 120��C
��3����Ӧ��II�з�����Ӧ�����ӷ���ʽΪ ��
��4���ӿ췴Ӧ��II�з�Ӧ���ʵĴ�ʩ�� ��д��һ�ִ�ʩ���ɣ��ȡ��ӷ�Ӧ��II�л��NaClO2 ��Ʒ��ʵ����������� ������ţ���ͬ������һ���ᴿ�IJ�������Ϊ ��
A������ B���ؽᾧ C������ D������Ũ�� E���������� F����ȴ�ᾧ G����ȡ��Һ
��5�����������п�ѭ��ʹ�õ�����Ϊ ������Ʒ��NaClO2��NaHSO4��� ���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�Ǿ����Դ���⣬��ˮ���������ۺ����þ�����Ҫ���塣
���������գ�
��1���ȼҵ��Ҫ��ʳ��Ϊԭ�ϡ�Ϊ�˳�ȥ�����е�Ca2+��Mg2+��SO42������ɳ���ɽ���������ˮ��Ȼ��������в�������ȷ�IJ���˳���� ��
�ٹ��ˣ��ڼӹ�����NaOH��Һ���ۼ�����������ܼӹ�����Na2CO3��Һ���ݼӹ�����BaCl2��Һ
a���ڢݢܢ٢� b���٢ܢڢݢ� c d���ݢڢܢ٢�
��2����ʵ�����п�������ȡ�ķ�����ȡ�壬��ѡ�õ��Լ���________________��������Ҫ������������____________________��
��3����������������ữ�����Cl2�����ʵ�ԭ���� ��
��4������II��Ӧ�����ӷ���ʽ__________________________________________��
��5����ˮ������������У��¶�Ӧ������80~90�棬�¶ȹ�����Ͷ����������� �������ԭ�� ��
��6��Mg(OH)2�����л���Ca(OH)2����ѡ��__________��Һ����ϴ�ӳ�ȥ����ֱ�Ӽ���Mg(OH)2�õ�MgO���ٵ������MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��_______��ѡ�ͬ�⡱������ͬ�⡱����˵���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���������̿�MnO2Լ70����Al2O3������п��ZnSԼ80����FeS������ͬ����MnO2��Zn���ɵ��ԭ�ϣ���
��֪����A��MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O  MnO2+Zn+2H2SO4��
MnO2+Zn+2H2SO4��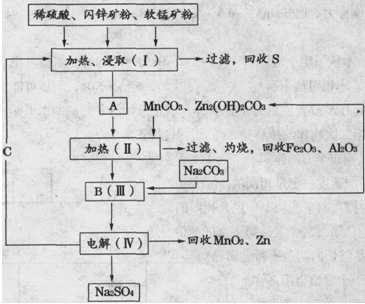
��1��A�����ڻ�ԭ������� ��
��2��MnCO3��Zn2��OH��2CO3�������� ������Ҫ���ȵ�ԭ���� ��C�Ļ�ѧʽ�� ��
��3�����з��������ӷ���ʽΪ �� ��
��4����������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±����������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ����
| | NaNO3 | KNO3 | NaCl | KCl |
| 10�� | 80.5 | 21.2 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��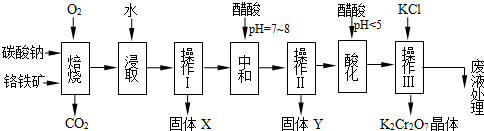
��֪����4FeO��Cr2O3+ 8Na2CO3+ 7O2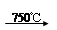 8Na2CrO4 + 2 Fe2O3 + 8CO2����
8Na2CrO4 + 2 Fe2O3 + 8CO2����
��Na2CO3 + Al2O3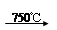 2NaAlO2 + CO2������ Cr2O72��+ H2O
2NaAlO2 + CO2������ Cr2O72��+ H2O 2CrO42�� + 2H+
2CrO42�� + 2H+
��1������X����Ҫ����_________����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ��__________����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH��5����Ŀ����_________________________________��
| ���� | �ܽ��/(g/100gˮ) | ||
| 0��C | 40��C | 80��C | |
| KCl | 28 | 40.1 | 51.3 |
| NaCl | 35.7 | 36.4 | 38 |
| K2Cr2O7 | 4.7 | 26.3 | 73 |
| Na2Cr2O7 | 163 | 215 | 376 |
 ), �÷�Ӧ����������Cr2O72-�Ĵ��ڡ�д����Ӧ�����ӷ���ʽ�� ��
), �÷�Ӧ����������Cr2O72-�Ĵ��ڡ�д����Ӧ�����ӷ���ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ�ֺ�����ﮡ��ܵ����͵��Ӳ��ϣ������в����ķ��������ɹۣ������е����Խ�����������ʽ���ڣ�����Co2O3·CoO����ʽ���ڣ������������ĵ����˫�棻﮻��������С�
�ӷ����л��������ܣ�CoO���Ĺ����������£�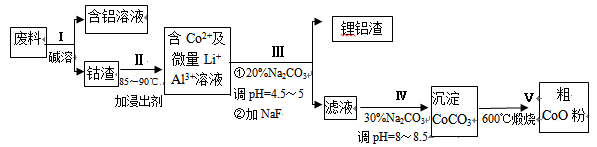
��1������I�в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ ��
��2������II�м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܡ�������ܵĻ�ѧ��Ӧ����ʽΪ��������ֻ��һ������� ����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ�ʹ�ҵ�����в������ᣬ��ӷ�Ӧԭ������������������ܵ���Ҫԭ��_______________��
��3�����̢�õ����������Ҫ�ɷ���LiF��Al(OH)3��̼������Һ�ڲ���Al(OH)3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ________________________��
��4��̼������Һ�ڹ���III��IV����������������ͬ����д���ڹ���IV�����������
____________________________________________________________��
��5����Na2CO3��Һ�д��ڶ������ӣ����и�����Ũ�ȹ�ϵ��ȷ����______������ţ���
| A��c(Na+) = 2c(CO32-) | B��c(Na+) > c(CO32-) > c(HCO3-) |
| C��c(OH-) > c(HCO3-) > c(H+) | D��c(OH-) - c(H+)��c(HCO3-) + 2c(H2CO3) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���й����ʵķ���ش��������⣺
��1������һƿA��B�Ļ��Һ����֪���ǵ��������±���
| ���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | �ܽ��� |
| A | ��11��5 | 198 | 1��11 | A��B���ܣ��Ҿ�������ˮ |
| B | 17��9 | 290 | 1��26 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���÷����ú��ʯ����Ҫ��Al2O3��SiO2������������Ʊ���ˮ��BAC��Al2(OH)nCl6-n�����������£�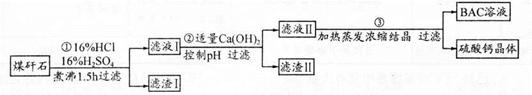
��1������ú��ʯ��Ŀ����______;����I����Ҫ�ɷ���______ (�ѧʽ)��
��2�����������еĹ����У���Һ������ɫ��Ϊ��ɫ����ʱ��Һ����ɫ����Ϊ______(�ѧʽ��;�����Һ�ֱ�Ϊ�ػ�ɫ��������ӷ�Ӧ����ʽΪ______;
����ٵ����װ���Ϸ��谲װһ�����ܣ������ܵ�������____________��
��3��������м���������Ca(OH)2������pH����Ŀ��:һ������BAC;����___________����֪BAC�ķ�ɢ�����Ӵ�С��1 nm?100 nm֮�䣬�ɴ�������ҺI��BAC����Һ�������������______;��Ca(OH)2��Һ����,����۵õ���BAC����ƫ�ͣ�д���÷�Ӧ�����ӷ���ʽ______��
��4����0.1 molAlCl3��ij�¶�����������ˮ������5�Gˮ������Al(OH)3��Һʱ����������a kJ��д���ù��̵��Ȼ�ѧ����ʽ____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com