
���й����ʵķ���ش��������⣺
��1������һƿA��B�Ļ��Һ����֪���ǵ��������±���
| ���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | �ܽ��� |
| A | ��11��5 | 198 | 1��11 | A��B���ܣ��Ҿ�������ˮ |
| B | 17��9 | 290 | 1��26 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧʵ���Ҳ����ķ�Һ�к��д�������Ⱦ���������ʣ�Ϊ�˱�����������Щ��Һ���뾭����������ŷš�ij��ѧʵ���Ҳ����ķ�Һ�к������ֽ������ӣ�Fe3+��Cu2+����ѧС�����������ͼ��ʾ�ķ����Է�Һ���д������Ի��ս���������������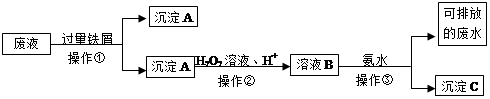
��1�������ٵ������� ��
��2������A�к��еĽ��������� ��
��3�����������������·�����Ӧ�����ӷ���ʽ ��
��4��������ҺB�к��еĽ��������ӳ��õ��Լ��� ��
��5���������з�����Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�廯��(CaBr2��2H2O)��һ�ְ�ɫ���壬������ˮ���к�ǿ����ʪ�ԣ��ǹ���ֽ��Ȫˮ����������Ҫ�ɷ֣���ҽҩ������������˥���ȵ�ҩ�Ҳ������ѧ�������ù�ҵ����ʯ����������Al3+��Fe3+�����ʣ��Ʊ��廯�Ƶ���Ҫ��������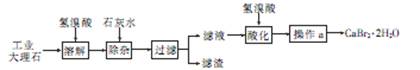
�ش���������
��1���ܽ�ʱ��������Ҫ��Ӧ�����ӷ���ʽΪ
��2�����Ӳ��������Һ��pHԼΪ8��0��Ŀ���� ��
��3����Һ���������ữ��Ŀ���� ������a��Ҫ���� �� ����
��4���Ƶõ��廯�ƾ������ͨ�����²���ⶨ�䴿�ȣ�
�ٳ�ȡ5��00g�廯�ƾ�����Ʒ�����ܽ⣻�۵�������Naa2CO3��Һ����ַ�Ӧ����ˣ��ܺ�ɡ���ȴ���ݳ��������õ�2�� 00 g̼��ƣ�����Ʒ�Ĵ���Ϊ
��5���廯�ƾ����������Ӻ����ӵļ���
�ٽ������廯�ƾ�������ˮ�����������ữ��AgNO3��Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
�ڽ������廯�ƾ�������ˮ���μӲ�������Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
NiSO4��6H2O��һ����ɫ������ˮ�ľ��壬�㷺���ڻ�ѧ������������صȣ����ɵ�Ʒ������������⣬�����У�Cu��Zn��Fe��Cr�����ʣ�Ϊԭ�ϻ�á������������£�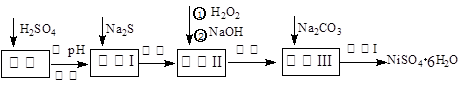
��1����Na2S��Ŀ���dz�ȥͭ��п�����ʣ���д����ȥCu2+�����ӷ���ʽ__________ __________
��2�� ��6%��H2O2ʱ���¶Ȳ��ܹ��ߣ�����Ϊ�� _____ ________ ��
��3�� ������������H2O2�������������NaOH����pHֵ3��4��Χ�������������������������������У�����������NaClO3���棬��д��������������Fe2+�����ӷ���ʽΪ��___________________________________________________________________________
��4��������������Һ�����Ҫ�ɷ��ǣ��� �������ѧʽ��
��5��������������¹��̣����ˣ��� �����Լ���ѧʽ���ܽ⣬ ��ϴ�ӻ�ò�Ʒ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��ʳ�γ���������Ca2����Mg2����Fe3����SO42�����������ӣ�ʵ�����ṩ���Լ����£�����Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��75%�Ҵ������Ȼ�̼��ʵ�����ᴿNaCl���������£�
��1������ȥ��Һ���е�Ca2����Mg2����Fe3����SO42�����ӣ�ѡ��A�������Ķ����Լ������μ�˳������Ϊi NaOH ii iii ���ѧʽ����
��2����д�������Լ�����ʱ������Ӧ�����ӷ�Ӧ����ʽ��
�����Լ�i�� ��
�����Լ�iii�� ��
��3��ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ� Ϊ �������ṩ���Լ���ѡ��
��4��ʵ�����õ�����������ʵ���Ũ��Ϊ0��400mol/L����ʵ����ijŨ�����Լ�ƿ�ϵ��й��������£�
��������Ũ��������ʵ������Ũ�ȵ�ϡ����480mL��
��������Ҫ�IJ��������� �����������ƣ�
������ȡ��Ũ��������Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�����ĩ,���п��ܺ���Na2CO3��NaCl��Na2SO4��Ba(NO3)2��K2CO3��K2SO4�е�һ�ֻ��֣��ְ����в������ʵ�顣
��1�����÷�ĩ����ˮ����ɫ��Һ�Ͱ�ɫ������
��2�����˳��ij����м���ϡ���ᣬ�в��ֳ����ܽ⣬ͬʱ������ɫ���塣
��3��ȡ��Һ����ɫ��Ӧ����֤����Һ�к�Na+������K+��
�����������ƶϣ�
��1���û������һ������ ���� ��һ�������� �����ܺ��� ��
��2����Ҫ�Կ��ܺ��е����ʽ��м��飬��β�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪʵ������ȡ����ˮ��װ��ʾ��ͼ������ͼʾ�ش��������⡣
��1��ͼ�е��������ԵĴ����ǣ�________________________ ��_________________________________��
��2��B������������_______________��
��3��ʵ��ʱA�г�������������ˮ�⣬�����������_______����������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ﮣ�LiFePO4������Ϊ������ǰ;������ӵ���������ϡ�ij��ҵ���ø�������Һ����������ﮣ������˴�������������Һһ����;��������Ҫ�������£�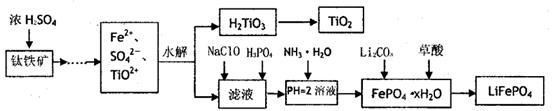
��֪��H2LiO3����������ˮ�����ʡ�
(1)��������Ũ���ᴦ��֮ǰ����Ҫ���飬��Ŀ��
(2)TiO2+ˮ������H2TiO3�����ӷ���ʽ
(3)����NaClO������Ӧ�����ӷ���ʽ
(4)��ʵ���У�����Һ�й��˳�H2TiO3��������Һ���ǣ�Ӧ��β��� ��
(5)Ϊ�ⶨ�����������ĺ�����ijͬѧȡ��Ũ����ȴ�������Һ����ʱ�������е�����ȫ��ת��Ϊ���������ӣ�����ȡKMnO4��Һ�춨Fe2+�ķ�����(������KMnO4���������ʷ�Ӧ)�ڵζ������У���δ�ñ�Һ��ϴ�ζ��ܣ���ʹ�ⶨ��� �� (�ƫ�ߡ�ƫ�͡���Ӱ�족)���ζ��յ������ ���ζ�����ʱ����ȡa g������������cmol/LKMnO4��Һ�ζ�������VmL������Ԫ�ص����������ı���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ױ��������Ʊ�������ķ�Ӧԭ�����£� + 2KMnO4
+ 2KMnO4
 +KOH+2MnO2��+H2O
+KOH+2MnO2��+H2O + HCl
+ HCl
 +KCl
+KCl
ʵ��ʱ��һ�����ļױ���KMnO4��Һ����ͼ1װ���У���100 ��ʱ�� ��Ӧһ��ʱ�䣬��ֹͣ��Ӧ�������������̷����������ͻ���δ��Ӧ�ļױ���


ͼ1��������װ�� ͼ2����װ��
��ʵ������ʵ�ֲ���������IJ��������� ���ձ��������������Ϊ ��
�������Һ����ɫ��Ҫ�ȼ���������أ�Ȼ���ټ���Ũ�����ữ�����˲�������ֵ�Σ���� ��
���ڲ������У�����ǰ�������ȴ��Һ����ԭ���� ����ͼ2��ʾ������ϣ�Ӧ�ȶϿ� ֮�����Ƥ�ܡ�
�ȴ��Ȳⶨ����ȡ1.220 g��Ʒ�����100 mL��Һ��ȡ����25.00 mL��Һ�����еζ� ������KOH���ʵ���Ϊ2.4��10��3 mol����Ʒ�б�������������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com