
�ױ��������Ʊ�������ķ�Ӧԭ�����£� + 2KMnO4
+ 2KMnO4
 +KOH+2MnO2��+H2O
+KOH+2MnO2��+H2O + HCl
+ HCl
 +KCl
+KCl
ʵ��ʱ��һ�����ļױ���KMnO4��Һ����ͼ1װ���У���100 ��ʱ�� ��Ӧһ��ʱ�䣬��ֹͣ��Ӧ�������������̷����������ͻ���δ��Ӧ�ļױ���


ͼ1��������װ�� ͼ2����װ��
��ʵ������ʵ�ֲ���������IJ��������� ���ձ��������������Ϊ ��
�������Һ����ɫ��Ҫ�ȼ���������أ�Ȼ���ټ���Ũ�����ữ�����˲�������ֵ�Σ���� ��
���ڲ������У�����ǰ�������ȴ��Һ����ԭ���� ����ͼ2��ʾ������ϣ�Ӧ�ȶϿ� ֮�����Ƥ�ܡ�
�ȴ��Ȳⶨ����ȡ1.220 g��Ʒ�����100 mL��Һ��ȡ����25.00 mL��Һ�����еζ� ������KOH���ʵ���Ϊ2.4��10��3 mol����Ʒ�б�������������Ϊ ��
�ŷ�Һ©�� ���� �ƹ����ĸ�������������ᣬ��������
�ǵõ��ϴ�ı����ᾧ�������ڳ��� �����úͰ�ȫƿ ��96%��ÿ��2�֣���12�֣�
��������������Ų���IΪ��Һ������������IJ��������з�Һ©�����ձ�������IIΪ�����������ɫҺ��A��
��Ϊ�˱�������ĸ�������������ᣬ�����ж���������Ӧ���ȼ���������س�ȥ�����ĸ�����أ�Ȼ���ټ���Ũ�����ữ��
��Ϊ�˵õ��ϴ�ı����ᾧ�������ڳ��ˣ�Ӧ���ڳ���ǰ�������ȴ��Һ��Ϊ�˱���ܵ����ˮ�ڸ�ѹ�»��������ڳ�����ϣ�Ӧ�ȶϿ������úͰ�ȫƿ��
�ȸ��ݡ�1 ��1KOH�������Ʒ�б�������������Ϊ
��1KOH�������Ʒ�б�������������Ϊ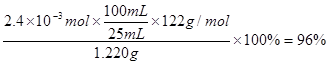 ��
��
���㣺���⿼�黯ѧ�ۺ�ʵ�飨��Һ������ʵ�黷������ȫ������������ȣ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���й����ʵķ���ش��������⣺
��1������һƿA��B�Ļ��Һ����֪���ǵ��������±���
| ���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | �ܽ��� |
| A | ��11��5 | 198 | 1��11 | A��B���ܣ��Ҿ�������ˮ |
| B | 17��9 | 290 | 1��26 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���÷����ú��ʯ����Ҫ��Al2O3��SiO2������������Ʊ���ˮ��BAC��Al2(OH)nCl6-n�����������£�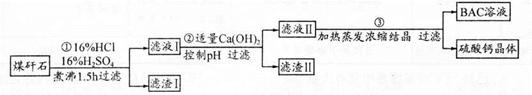
��1������ú��ʯ��Ŀ����______;����I����Ҫ�ɷ���______ (�ѧʽ)��
��2�����������еĹ����У���Һ������ɫ��Ϊ��ɫ����ʱ��Һ����ɫ����Ϊ______(�ѧʽ��;�����Һ�ֱ�Ϊ�ػ�ɫ��������ӷ�Ӧ����ʽΪ______;
����ٵ����װ���Ϸ��谲װһ�����ܣ������ܵ�������____________��
��3��������м���������Ca(OH)2������pH����Ŀ��:һ������BAC;����___________����֪BAC�ķ�ɢ�����Ӵ�С��1 nm?100 nm֮�䣬�ɴ�������ҺI��BAC����Һ�������������______;��Ca(OH)2��Һ����,����۵õ���BAC����ƫ�ͣ�д���÷�Ӧ�����ӷ���ʽ______��
��4����0.1 molAlCl3��ij�¶�����������ˮ������5�Gˮ������Al(OH)3��Һʱ����������a kJ��д���ù��̵��Ȼ�ѧ����ʽ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
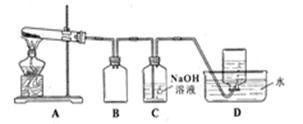
��12�֣�1,2 ����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18 g��cm��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1,2 ���������顣���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ��(���渲������ˮ)��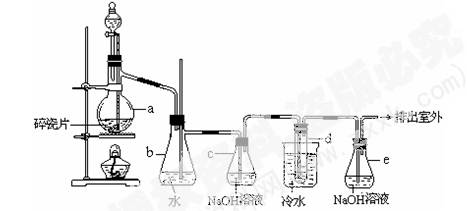
��д���пհף�
��1��д���������Ʊ�1,2-���������������ѧ��Ӧ����ʽ��_ ��_____________________��
��2����ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����____________________________________________��
��3������c��NaOH��Һ�������ǣ�________________________________________��
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������ࡣ���װ�õ�������û�����⣬�Է�������ܵ�ԭ�� ______________�� _____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������Ҫ�Ʊ��������Ȼ��ؾ��塣���к�����KBr��K2SO4���Ȼ�����Ʒ��������ͼ��ʾ��ʵ�鷽�������ᴿ��
��1������I������ ������������IJ��������оƾ��ƺ� ��
��2���������ᱵ�������Ȼ������Ƿ���У���˵�����ɡ�
��
��3����ʵ�����ù���ס��ҵ������ֱ�ΪW1g��W2g������Ʒ��KBr��������������ʽΪ ��
��4��ijͬѧ�Ը�ʵ�鷽��������ɣ�����Ϊ�������Ȼ�����Һ�������ƣ�Ӧ��������Ȼ�����Һ�����㰴����˼·��д��ʵ������ͼ�������Լ������ò���Ļ�ѧʽ����Ӧ���������ƣ�A ��B ����ҺC �������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣��Ȼ�����Ʒ�к�����̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ�����Һ����ͼ��ʾ������в�����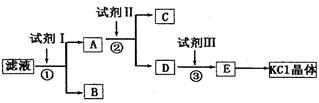
�ش��������⣺
(1)��ʼʱ��Һ��pH_____7������ڡ���С�ڡ����ڡ�������ԭ����__________________�������ӷ���ʽ��ʾ����
(2)�Լ�I�Ļ�ѧʽΪ______�����з�����Ӧ�����ӷ���ʽΪ_____________________��
(3)�Լ�II�Ļ�ѧʽΪ_____�����м����Լ�II��Ŀ����_________________________��
(4)�Լ�III��������_____�����з�����Ӧ�����ӷ���ʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�����ʯ�к��е���Ҫ��������������������ij��ѧ��ȤС���ô���ʯΪԭ����ȡ��ȫ��ɱ�����������Ƶ���Ҫ���̣�
��ش��������⣺
��1���Լ�A�������� ��
��2������I��Ŀ���� ��
��3����ʵ����Ҫ���ʹ�ò���������ʵ������в������������� ��
��4��д����Ӧ��������CaO2��8H2O�Ļ�ѧ����ʽ�� ��
��5���Ƶõ�CaO2��һ�㺬��CaO�û�ѧ��ȤС��ͨ��ʵ��ⶨ�Ƶõ���Ʒ��CaO2�ĺ���������0.6g��Ʒ����ƿ�У�Ȼ����������Ũ��Ϊ2.00mol?L-1������20.00mL������Ũ��Ϊ2.00mol?L-1������������Һ�ζ���ƿ�е���Һ����������������Һ11.00mL������Ʒ��CaO2����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��̽��AgNO3�������Ժ����ȶ��ԣ�ij��ѧ��ȤС�����������ʵ�顣
��. AgNO3��������
����������˿����AgNO3��Һ�У�һ��ʱ�����˿ȡ����Ϊ������Һ��Fe�������������Һ�е�Ag+��������������ʵ�顣��ѡ���Լ���KSCN��Һ��NaOH��Һ����ˮ��KMnO4��Һ��
��������±���
| ���� | ���� | ���� |
| ȡ��������Ag+�����Һ���Թ��У����� ���� | | ����Fe3+ |
| ȡ��������Ag+�����Һ���Թ��У�����1~2��KMnO4��Һ���� | | ����Fe2+ |
| ʵ���� | ���� | ���� |
| a | ����������ˮ���� | ��ɫ���岻�ܽ� |
| b | ��������ϡ���ᣬ�� | ��ɫ�����ܽ⣬����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪ij����������ȡҺ�г�Fe3���⣬������һ����Mg2����Al3��������ƺ���ʵ�����ý�ȡҺ�е�Mg2����Al3����
��ѡʵ����Ʒ���Լ����ձ����Թܡ����������ιܡ�����Ƭ��pH��ֽ����ȡҺ��20% KSCN��0.1 mol��L��1NaOH��6 mol��L��1NaOH��0.1 mol��L��1HCl������ˮ��
��Ҫʱ�ɲο���
| ������ | ��ʼ����ʱ��pH | ������ȫʱ��pH |
| Mg(OH)2 | 9.6 | 11.1 |
| Fe(OH)3 | 2.7 | 3.7 |
| ʵ����� | Ԥ������ͽ��� |
| ����1�� | |
| ����2�� | |
| ����3�� | |
| ���� | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com