��2011?����һģ��[��ѧ�뼼��]���Ṥҵ����Ӧ�����ۺϾ���Ч�����⣮
��1�����������ĸ�������ѡ��һ���½�һ�����᳧������Ϊ��ַ��ѡ��
C
C
�Ľ�������ѡ��ı�ţ�
A���зḻ��������Դ�ij��� B��������������γ���
C��������������Ĺ�ҵ���� D���˿ڳ��ܵ��Ļ�����ҵ���ij���
��2���ݲ��㣬�Ӵ�������������У�����Ӧ�ȶ�δ�����ã���ÿ����1t 98%����������3.6��10
5kJ��������ͨ�������жϣ�����Ӧ��SO
2��g��+
O
2?SO
3 ��H=-98.3kJ?mol
-1�ų��������������������еõ�������ã���ÿ����1t98%����ֻ������ṩ���������������
6.23��105
6.23��105
ǧ��������H
2SO
4��Ħ������Ϊ98g?mol
-1��
��3��CuFeS
2�ǻ��������һ�ɷ֣�����ʱ��CuFeS
2ת��ΪCuO��Fe
2O
3��SO
2���÷�Ӧ�Ļ�ѧ����ʽΪ
4CuFeS
2+13O
24CuO+2Fe
2O
3+8SO
24CuFeS
2+13O
24CuO+2Fe
2O
3+8SO
2��
��4�������᳧����¯�ų��Ŀ����к���Fe
2O
3��CuO��CuSO
4����CuO��SO
3�ڷ���¯�л��϶��ɣ�����������ͭ���������������¯�¶Ȳ�ͬ���仯�����±���
| ����¯�¶�/�� |
600 |
620 |
640 |
660 |
| ������CuSO4����������/% |
9.3 |
9.2 |
9.0 |
8.4 |
��֪CuSO
4�ڵ���660��ʱ����ֽ⣬���Ҫ�����ϱ���CuSO
4�������������¶����߶����͵�ԭ��
SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ�����¶����ߣ�ƽ�����ƣ�SO3���ʵ������٣�����CuSO4�������٣����¶����ߣ�SO3���ʵ������٣���CuSO4�������٣�
SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ�����¶����ߣ�ƽ�����ƣ�SO3���ʵ������٣�����CuSO4�������٣����¶����ߣ�SO3���ʵ������٣���CuSO4�������٣�
��




 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
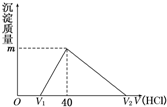 ��2011?������ģ����һ�����������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬�õ�20mL OH-Ũ��Ϊ1mol/L����Һ��Ȼ����1mol/L������ζ���������������������������ϵ��ͼ��ʾ��������ѡ����ȷ���ǣ�������
��2011?������ģ����һ�����������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬�õ�20mL OH-Ũ��Ϊ1mol/L����Һ��Ȼ����1mol/L������ζ���������������������������ϵ��ͼ��ʾ��������ѡ����ȷ���ǣ�������