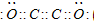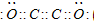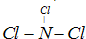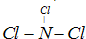����A��B��C��D��E����ǿ����ʣ�������ˮ�пɵ�������������ӣ��������Ӳ��ظ�����
| ������ |
H+��Na+��Al3+��Ag+��Ba2+ |
| ������ |
OH-��Cl-��CO32-��NO3-��SO42- |
��֪����A��B����Һ�ʼ��ԣ�C��D��E��Һ�����ԣ�
��A��Һ��E��Һ��Ӧ�����������г���������A��Һ��C��Һ��Ӧֻ������������������������ͬ����
��D��Һ������������Һ��Ӧ���ܲ���������Cֻ����D��Ӧ����������
�Իش��������⣺
��1����C��Һ��μ��������������ʵ�����Ũ�ȵ�A��Һ�У���Ӧ����Һ�и�������Ũ���ɴ�С��˳��Ϊ��
c��Na+����c��C1-����c��HCO3-����c��OH-����c��H+����c��CO32-��
c��Na+����c��C1-����c��HCO3-����c��OH-����c��H+����c��CO32-��
��
��2��д��E��Һ�������B��Һ��Ӧ�����ӷ���ʽ��
2Al3++3SO42-+3Ba2++8OH-�T2AlO2-+4H2O+3BaSO4��
2Al3++3SO42-+3Ba2++8OH-�T2AlO2-+4H2O+3BaSO4��
��
��3����֪��NaOH��aq��+HNO
3��aq��=NaNO
3��aq��+H
2O��l������H=-a KJ?mol
-1��
��д��B��C��ϡ��Һ��Ӧ���Ȼ�ѧ����ʽ
OH
-��aq��+H
+��aq��=H
2O��1����H=-aKJ/mol��
Ba��OH��
2��aq��+2HC1��aq��=
BaC1
2��aq��+H
2O��1����H=-aKJ/mol
��Ba��OH��
2��aq��+2HC1��aq��=BaC1
2��aq��+H
2O��1����H=-2aKJ/mol
OH
-��aq��+H
+��aq��=H
2O��1����H=-aKJ/mol��
Ba��OH��
2��aq��+2HC1��aq��=
BaC1
2��aq��+H
2O��1����H=-aKJ/mol
��Ba��OH��
2��aq��+2HC1��aq��=BaC1
2��aq��+H
2O��1����H=-2aKJ/mol
��
��4����100mL 0.1mol?L
-1E��Һ�У���μ���35mL 2mol?L
-1NaOH��Һ�����յõ��������ʵ���Ϊ
0.01mol
0.01mol
��




 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��