
�����й�˵����ȷ����(����)
A�����ں�������ϸ���һЩͭ�飬����Լ���������ǵĸ�ʴ
B��2NO(g)��2CO(g)===N2(g)��2CO2(g)�ڳ��������Է����У���÷�Ӧ�Ħ�H>0
C������0.1 mol��L��1Na2CO3��Һ��CO ��ˮ��̶Ⱥ���Һ��pH������
��ˮ��̶Ⱥ���Һ��pH������
D�������������Ҵ���������Ӧ(��H<0)����������Ũ���Ტ���ȣ��÷�Ӧ�ķ�Ӧ���ʺ�ƽ�ⳣ��������
C��[����] ���ֵ�����Ǹ�����������ͭ���ã���������ϸ���ͭ����γ�ԭ��أ���������������ٺ�����ǵĸ�ʴ��A�����B��ӦΪ���������С�ķ�Ӧ����Ӧ��S��0������Ӧ���Է����У���H��T��S��0���ʷ�Ӧ�Ħ�H��0��B��������������ˮ�ⷴӦΪ���ȷ�Ӧ�������¶�������CO ��ˮ��̶ȣ���Һ��c(OH��)������ҺpH����C����ȷ��Ũ������������Ӧ�Ĵ�������������Ũ����ʹ��Ӧ�������÷�ӦΪ���ȷ�Ӧ��һ��ʱ�������ϵ�¶����ߣ�ƽ�������ƶ���ƽ�ⳣ����С��D�����
��ˮ��̶ȣ���Һ��c(OH��)������ҺpH����C����ȷ��Ũ������������Ӧ�Ĵ�������������Ũ����ʹ��Ӧ�������÷�ӦΪ���ȷ�Ӧ��һ��ʱ�������ϵ�¶����ߣ�ƽ�������ƶ���ƽ�ⳣ����С��D�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��
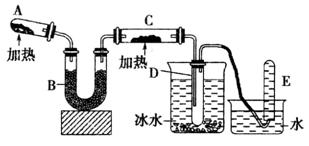
�ش��������⣺
(1)A�����ɰ�����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
(2)B�м���ĸ������_________(�����)��Ũ�������ˮ�Ȼ��� �ۼ�ʯ�ң�
(3)��֤����������ͭ��Ӧ�������C��______________����D������ɫҺ�����ɣ�
���ʵ�����D����ɫҺ�ijɷ֣�ȡ����Һ�����Թ��У���������________��ĩ������Ϊ___________________��
(4)д������������ͭ��Ӧ�Ļ�ѧ����ʽ___________________________�����ռ���2.24L(STP)����������ת�Ƶ�����Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
(1)��֪AX3���۵�ͷе�ֱ�Ϊ��93.6 ���76 �棬AX5���۵�Ϊ167 �档����ʱAX3������X2��Ӧ����1 mol AX5���ų�����123.8 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
(2)��ӦAX3(g)��X2(g)AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��

����ʽ����ʵ��a�ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)��______________________��
��ͼ��3��ʵ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)�ɴ�С�Ĵ���Ϊ____________(��ʵ�����)����ʵ��a��ȣ���������ı��ʵ���������ж������ǣ�b________________________________________________��c____________________________________________��
����p0��ʾ��ʼʱ��ѹǿ��p��ʾƽ��ʱ��ѹǿ������ʾAX3��ƽ��ת���ʣ������ı���ʽΪ______________��ʵ��a��c��ƽ��ת���ʣ���aΪ________����cΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
(1)��֪AX3���۵�ͷе�ֱ�Ϊ��93.6 ���76 �棬AX5���۵�Ϊ167 �档����ʱAX3������X2��Ӧ����1 mol AX5���ų�����123.8 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
(2)��ӦAX3(g)��X2(g)  AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��

����ʽ����ʵ��a�ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)��______________________��
��ͼ��3��ʵ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)�ɴ�С�Ĵ���Ϊ____________(��ʵ�����)����ʵ��a��ȣ���������ı��ʵ���������ж������ǣ�b________________________________________________��c____________________________________________��
����p0��ʾ��ʼʱ��ѹǿ��p��ʾƽ��ʱ��ѹǿ������ʾAX3��ƽ��ת���ʣ������ı���ʽΪ______________��ʵ��a��c��ƽ��ת���ʣ���aΪ________����cΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10 L�����ܱ������г���X(g)��Y(g)��������ӦX(g)��Y(g)  M(g)��N(g)������ʵ���������±���
M(g)��N(g)������ʵ���������±���
| ʵ�� ��� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ||
| n(X) | n(Y) | n(M) | |||
| �� | 700 | 0.40 | 0.10 | 0.090 | |
| �� | 800 | 0.10 | 0.40 | 0.080 | |
| �� | 800 | 0.20 | 0.30 | a | |
| �� | 900 | 0.10 | 0.15 | b | |
����˵����ȷ����(����)
A��ʵ����У���5 minʱ���n(M)��0.050 mol����0��5 minʱ���ڣ���N��ʾ��ƽ����Ӧ����v(N)��1.0��10��2 mol��L��1��min��1
B��ʵ����У��÷�Ӧ��ƽ�ⳣ��K��2.0
C��ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ60%
D��ʵ����У��ﵽƽ��ʱ��b>0.060
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ�Ϊ1.00 L�������У�ͨ��һ������N2O4��������ӦN2O4(g)2NO2(g)�����¶����ߣ�����������ɫ���
�ش��������⣺
(1)��Ӧ�Ħ�H________0(����ڡ���С�ڡ�)��100 ��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0��60 sʱ�Σ���Ӧ����v(N2O4)Ϊ________mol��L��1��s��1����Ӧ��ƽ�ⳣ��K1Ϊ________��
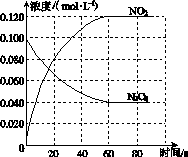
(2)100 ��ʱ��ƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.002 0 mol��L��1��s��1��ƽ�����ʽ��ͣ���10 s�ִﵽƽ�⡣
��T________100 ��(����ڡ���С�ڡ�)���ж�������____________________________��
����ʽ�����¶�Tʱ��Ӧ��ƽ�ⳣ��K2��_______________________________________
________________________________________________________________________��
(3)�¶�Tʱ��Ӧ��ƽ�����Ӧ�������ݻ�����һ�룬ƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����ж�������__________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1200��ʱ����Ȼ���������лᷢ�����з�Ӧ
H2S��g��+ 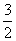 O2(g)=SO2(g)+H2O(g) ��H1
O2(g)=SO2(g)+H2O(g) ��H1
2H2S(g)+SO2(g)=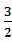 S2(g)+2H2O(g) ��H2
S2(g)+2H2O(g) ��H2
H2S(g)+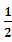 O2(g)=S(g)+H2O(g) ��H3
O2(g)=S(g)+H2O(g) ��H3
2S(g) =S2(g) ��H4
���H4����ȷ����ʽΪ
A.��H4��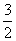 ����H1����H2��3��H3�� B.��H4��
����H1����H2��3��H3�� B.��H4��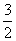 ��3��H3����H1����H2��
��3��H3����H1����H2��
C.��H4��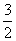 ����H1����H2��3��H3�� D.��H4��
����H1����H2��3��H3�� D.��H4��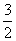 ����H1����H2��3��H3��
����H1����H2��3��H3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
SF6��һ�������ľ�Ե���壬���ӽṹ��ֻ����S-F������֪��1molS(s)ת��Ϊ��̬��ԭ����������280kJ,����1molF-F ��S-F�������յ������ֱ�Ϊ160kJ��330kJ����S(s)��3F2(g)=SF6(g)�ķ�Ӧ�ȡ�HΪ
A. -1780kJ/mol B. -1220 kJ/mol
C.-450 kJ/mol D. +430 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ����CO��CO2��H2��ˮ��������������ɵĻ�����壬Ҫ֤�����к���CO�� H2����ѡ�õ�������ҩƷ���£�
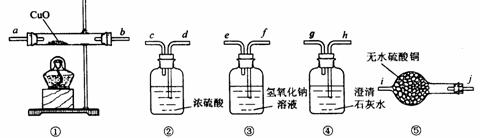
��1��ʵ��ʱ������װ�õ�˳���� �����ܽӿڵ�˳����
��2��װ �â��ڷ�����Ӧ�Ļ�ѧ����ʽ��
�â��ڷ�����Ӧ�Ļ�ѧ����ʽ��
��3��֤����������к���������ʵ��������
��4��֤����������к���CO��ʵ��������
��5��װ�âڵ������� ��װ�âܵ������� 
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com