��2011?�Ϻ�ģ�⣩�йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ�ã��г�װ��������ʡ�ԣ�����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н��ࣨ��Ъ�ԣ�ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺
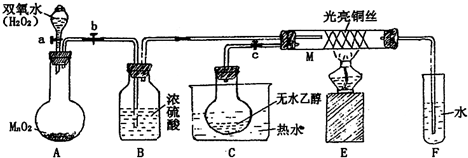
��1��A�з�����Ӧ�Ļ�ѧ����ʽ��
��B�����ã�
����O2
����O2
��C����ˮ�����ã�
C����ˮʹD���Ҵ���Ϊ��������M�вμӷ�Ӧ
C����ˮʹD���Ҵ���Ϊ��������M�вμӷ�Ӧ
��
��2��M�������ķ�Ӧ�Ļ�ѧ����ʽΪ��
��
��3����M���пɹ۲쵽������
���Ȳ��ֵ�ͭ˿���ڼ�Ъ�Եع��������������ֱ�ڣ���������
���Ȳ��ֵ�ͭ˿���ڼ�Ъ�Եع��������������ֱ�ڣ���������
�����п���ʶ����ʵ������д���
�μ�
�μ�
����μӡ����μӡ����˻�ѧ��Ӧ����������ʶ���������������Ҫһ����
�¶�
�¶�
��
��4����֤�Ҵ�����������Լ���
����������ͭ����Һ
����������ͭ����Һ
����д����Ӧ�Ļ�ѧ����ʽ
CH
3CHO+2Cu��OH��
2CH
3COOH+Cu
2O��+2H
2O
CH
3CHO+2Cu��OH��
2CH
3COOH+Cu
2O��+2H
2O
��
��5�����Թ�F���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����
CH3COOH
CH3COOH
��Ҫ��ȥ�����ʣ������ڻ��Һ�м���
c
c
����д��ĸ����
a���Ȼ�����Һ������b�������� c��̼��������Һ ����d�����Ȼ�̼��

 ��2011?�Ϻ�����ͼ��ʵ������ȡHCl����NaCl+H2SO4��Ũ��
��2011?�Ϻ�����ͼ��ʵ������ȡHCl����NaCl+H2SO4��Ũ��

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
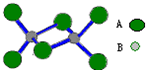 ��2011?�Ϻ�ģ�⣩��ͼ��ij��������Ķ��۷��ӣ��÷�����A��B����Ԫ�ض��ǵ������ڵ�Ԫ�أ�����������ԭ�ӵ��������Ӷ��ﵽ8�����ӵ��ȶ��ṹ������˵������ȷ���ǣ�������
��2011?�Ϻ�ģ�⣩��ͼ��ij��������Ķ��۷��ӣ��÷�����A��B����Ԫ�ض��ǵ������ڵ�Ԫ�أ�����������ԭ�ӵ��������Ӷ��ﵽ8�����ӵ��ȶ��ṹ������˵������ȷ���ǣ������� ��2011?�Ϻ���ʵ������ȡ�����������װ������ͼ��ʾ�������������������գ�
��2011?�Ϻ���ʵ������ȡ�����������װ������ͼ��ʾ�������������������գ�