
����Ŀ����1��ijͬѧΪ̽������KMnO4��Һ��H2C2O4�����ᣬ��Ԫ���ᣩ��Һ�ķ�Ӧ���̣���������ʵ�顣������������⣺
��д������KMnO4��Һ��H2C2O4�����ӷ���ʽ___________________________________��
������100mL0.0400mol��L-1��H2C2O4��Һ�����õ�������ƽ��ҩ�ס��ձ�����Ͳ���������������⣬�������õ��IJ���������_______________________________________��
�۽�KMnO4��Һ��ε���һ�����������H2C2O4��Һ�У��¶���ͬ����������¼���������£�
����KMnO4��Һ�Ĵ��� | KMnO4��Һ��ɫ��ȥ�����ʱ�� |
�ȵ����1�� | 60s |
��ɫ���ٵ����2�� | 15s |
��ɫ���ٵ����3�� | 3s |
��ɫ���ٵ����4�� | 1s |
�����KMnO4��Һ��ɫʱ��仯�Ŀ���ԭ��___________________________________��
��2��![]() ��
��![]() ����Һ�п��ת���������£���ʼŨ��Ϊ1.0 mol��L-1��Na2CrO4��Һ��
����Һ�п��ת���������£���ʼŨ��Ϊ1.0 mol��L-1��Na2CrO4��Һ��![]() ��c(H+)�ı仯��ͼ��ʾ
��c(H+)�ı仯��ͼ��ʾ

�������ӷ���ʽ��ʾ��Һ��![]() ��
��![]() ��ת����Ӧ_________��
��ת����Ӧ_________��
����ͼ��֪����Һ���Լ�С�� ![]() ��ƽ��ת����_________���������С�����䡱����
��ƽ��ת����_________���������С�����䡱����
�������¶ȣ���Һ��![]() ��ƽ��ת���ʼ�С����÷�Ӧ�Ħ�H_________0������ڡ���С�ڡ����ڡ�����
��ƽ��ת���ʼ�С����÷�Ӧ�Ħ�H_________0������ڡ���С�ڡ����ڡ�����
���𰸡� ![]() 100mL����ƿ����ͷ�ι� ��Ӧ���ɵ�Mn2+�Է�Ӧ�д����ã���Mn2+��Ũ�ȴ��Ч������
100mL����ƿ����ͷ�ι� ��Ӧ���ɵ�Mn2+�Է�Ӧ�д����ã���Mn2+��Ũ�ȴ��Ч������ ![]() ��С С��
��С С��
����������1���ٲ��������Եĸ�����ط���������ԭ��Ӧ�����ɶ�����̼�������Ӻ�ˮ�����ӷ���ʽΪ��2MnO4-+5H2C2O4+6H+�T10CO2��+2Mn2++8H2O��������100mL0.0400mol��L-1��H2C2O4��Һ�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ����Ͳ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ��������������õ��������������÷ֱ��ǽ��衢���������Ի������õ��IJ���������100mL����ƿ����ͷ�ιܣ��۷�Ӧ���ɵ�Mn2+�Է�Ӧ�д����ã���Mn2+��Ũ�ȴ��Ч�����ã���2��������H+Ũ�ȵ�����CrO42-ת��ΪCr2O72-�����ӷ�ӦʽΪ��2CrO42-+2H+![]() Cr2O72-+H2O������Һ���Լ�С��ƽ��2CrO42-+2H+
Cr2O72-+H2O������Һ���Լ�С��ƽ��2CrO42-+2H+![]() Cr2O72-+H2O������У�CrO42-��ƽ��ת���ʼ�С���������¶ȣ���Һ��CrO42-��ƽ��ת���ʼ�С��ƽ�������ƶ���˵����������ȣ���÷�Ӧ�ġ�H��0��
Cr2O72-+H2O������У�CrO42-��ƽ��ת���ʼ�С���������¶ȣ���Һ��CrO42-��ƽ��ת���ʼ�С��ƽ�������ƶ���˵����������ȣ���÷�Ӧ�ġ�H��0��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и����е�����ָ����Һ��һ���ܴ����������(����)
A. c(FeCl3)��1.0 mol��L��1����Һ�У�HCO![]() ��Cl����H����Na��
��Cl����H����Na��
B. �����£���ˮ�������c(H��)��10��14mol��L��1��Һ�У�NH![]() ��K����CO
��K����CO![]() ��SO
��SO![]()
C. ��c(HCO![]() )��0.1 mol��L��1����Һ�У�NH
)��0.1 mol��L��1����Һ�У�NH![]() ��AlO
��AlO![]() ��Cl����NO
��Cl����NO![]()
D. �����£� 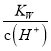 ��0.1 mol��L��1����Һ�У�K����NH3��H2O��SO
��0.1 mol��L��1����Һ�У�K����NH3��H2O��SO![]() ��NO
��NO![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼװ��(��)Ϊһ�ֿɳ���ص�ʾ��ͼ�����е����ӽ���Ĥֻ����K+ͨ�����õ�طŵ硢���Ļ�ѧ����ʽΪ2K2S2+KI3![]() K2S4 +3KI��װ��(��)Ϊ���ص�ʾ��ͼ�����պϿ���Kʱ���缫X������Һ�ȱ�졣��պ�Kʱ������˵������ȷ����( )
K2S4 +3KI��װ��(��)Ϊ���ص�ʾ��ͼ�����պϿ���Kʱ���缫X������Һ�ȱ�졣��պ�Kʱ������˵������ȷ����( )

A. K+������ͨ�����ӽ���Ĥ B. �缫A�Ϸ����ķ�ӦΪI3-+2e-=3I-
C. �缫Y�Ϸ����ķ�ӦΪ2Cl--2e-=Cl2�� D. ����0.1 molK+ͨ�����ӽ���Ĥ��X�缫�ϲ���1.12L����(��״��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ����(����)
A. ����ʯ��ˮ�������С�մ���Һ��Ӧ��Ca2����OH����HCO3-===CaCO3����H2O
B. ���Ը��������Һ��ϡ���ᷴӦ��5C2O42����2MnO4����16H��===2Mn2����10CO2��8H2O
C. AgCl������Һ�еμ�Na2S��Һ��2AgCl��S2��===Ag2S��2Cl��
D. NH4HCO3��Һ�������NaOH��Һ���ȣ�NH4+��OH��===NH3H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��CO2���������壬����NaOH��Һ���յõ�Na2CO3��NaHCO3��Na2CO3�׳ƴ����֪25��ʱ��CO32����һ��ˮ���ƽ�ⳣ��Kh=2��10-4mol/L������Һ��c(HCO3-)��c(CO32��)=20�� 1 ʱ����Һ��pH=______��
��2��Ϊ�˳�ȥ��������Ag2S���ɲ������·�������һ�����Ƶ������з���ʳ����Һ������������ʳ����Һ��ʹ���������Ӵ������γ�ԭ��أ���һ��ʱ�䣬���������Ϊ����ɫ�����ŵ�����������ζ���۲쵽��������ɫ��״�������ɣ���ԭ��ص�������ӦΪ______________________________������ͳ�������ζ�γɵ�ԭ�������ӷ���ʽ��ʾ��________________________________________��

��3��25 ������0.10 mol��L��1H2S��Һ�У�ͨ��HCl��������NaOH�����Ե�����ҺpH����ҺpH��c(S2��)��ϵ��ͼ(������Һ����ı仯��H2S�Ļӷ�)��
��pH��11ʱ����Һ�е�c(H2S)��c(HS��)��________mol��L��1��
��ij��Һ��0.040 mol��L��1M2����0.10 mol��L��1H2S������ҺpH��________ʱ��Mn2����ʼ������[��֪��Ksp(MS)��5.6��10��17]
��4��Na2S2O3��Һ����Ϊ��Һ�ⶨ���ʵ���ɡ�
I.ȡ3.92 gij�����������������ϡ���ᣬ�����Ƴ�100.0 mL��Һ��
II.ȡ10.00 mL������Һ����������KI��Һ���μӼ���ָʾ����
III.��0.2000 mol L-1��Na2S2O3����Һ�ζ����ظ�2��3�Σ�ƽ�����ı�Һ20.00mL��
��֪��I2+2S2O32-= S4O62-+2I-����
�ٲ���II ����ָʾ��������Ϊ____________���жϴﵽ�ζ��յ�IJ���������___________________��
�ڸ�����������Ļ�ѧʽΪ______________��
��5�������£���20 mL 0.2 mol /L H2A��Һ�еμ�0.2 mol /L NaOH��Һ���й��������ʵ����仯����ͼ�����������ߴ�������A2����H2A��HA��Ũ�ȱ仯�����ߣ�����ͼʾ����V(NaOH)��20 mLʱ����Һ��Na����HA���� A2���� H2A������Ũ�ȴ�С��ϵ��__________________________________����Һ��_______�ԡ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ���о������εij����ܽ�ƽ��ͳ���ת����ijͬѧ�������ʵ�顣
����1����2 mL 0.005 mol/LAgNO3��Һ�м���2 mL 0.005 mol/LKSCN��Һ�����á� | ���ְ�ɫ������ |
����2��ȡ1 mL�ϲ���Һ���Թ��У��μ�1��2 mol/LFe(NO3)3��Һ�� | ��Һ��Ϊ��ɫ�� |
����3������2����Һ�У���������5��3 mol/LAgNO3��Һ�� | ����a������Һ��ɫ��dz�� |
����4������1���µ���Һ�м���5��3mol/L KI��Һ�� | ���ֻ�ɫ������ |
��֪��25����Ksp(AgI����ɫ)= 8.3��1017 ��Ksp (AgSCN����ɫ )= 1.0��1012 ��
�ش��������⣺
��1������3������a��________________________________��
��2���ó����ܽ�ƽ��ԭ�����Ͳ���4��ʵ������________________��
��3����50 mL 0.005 mol/L��AgNO3��Һ�м���150 mL0.005 mol/L�� KSCN��Һ������Ϻ���Һ���Ϊ200mL������Һ��Ag+��Ũ��ԼΪ_____mol/L ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ի�����(FeS2)�������ƺ�������Һ��Ϸ�Ӧ�Ʊ������������壬����ˮ���ջ�ö���������Һ���ڴ˹�������Ҫ�������˵��¶ȣ����¶Ȳ���������Ӧ���ӣ�Ӱ������ClO2����Ĵ��ȣ��һ�Ӱ��ClO2����������ʡ������������ͼ��ʾ��

��ش��������⣺
(1)��ͼ��֪����Ӧʱ��Ҫ���Ƶ������¶���________ �棬�ﵽ��Ҫ���ȡ�����˴�ʩ��________��
(2)��֪���������е���Ԫ�������������±�ClO3��������SO42����д���Ʊ��������ȵ����ӷ���ʽ��____________________________________��
(3)ijУ��ѧѧϰС�����ԡ�m(ClO2)/m(NaClO3)����Ϊ����ClO2���ʵ�ָ�ꡣ��ȡNaClO3��Ʒ����6.0 g��ͨ����Ӧ�����տɵ�400 mL ClO2��Һ��ȡ��20 mL������37.00 mL 0.500 mol��L��1(NH4)2Fe(SO4)2��Һ��ַ�Ӧ������Fe2������0.0500 mol��L��1 K2Cr2O7����Һ�ζ����յ㣬����20.00 mL����Ӧԭ�����£�
4H����ClO2��5Fe2��===Cl����5Fe3����2H2O
14H����6Fe2����Cr2O72��===2Cr3����6Fe3����7H2O
�Լ���ClO2�ġ����ʡ�Ϊ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��K2Cr2O7��һ����Ҫ�Ļ���ԭ�ϡ��Ը�����(��Ҫ�ɷ�ΪFeO��Cr2O3,������Al2O3��Fe2O������)Ϊԭ���Ʊ�K2Cr2O7��һ�ֹ����������£�

��֪��(a) Cr2O72-+H2O![]() 2CrO42-+2H+
2CrO42-+2H+
(b)��ͬ�¶��¸����ʵ��ܽ��
�� �� | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 | |
�ܽ�ȣ�g/100g H2O�� | 0�� | 28 | 35.7 | 4.7 | 163 |
40�� | 40.1 | 36.4 | 26.3 | 215 | |
80�� | 51.3 | 38 | 70 | 376 | |
(c)����ٵ���Ҫ��ӦΪ��FeOCr2O3+Na2CO3+NaNO3![]() Na2CrO4+Fe2O3+CO2+NaNO2��δ��ƽ����
Na2CrO4+Fe2O3+CO2+NaNO2��δ��ƽ����
��1�� ������1���� ������2������Ҫ�ɷֱַ���_________��_________(�ѧʽ).
д���۵����ӷ�Ӧ����ʽ________________________________________;
������е�PH��ѡ�������Լ�__________��
A��NH3 B�� KOH C��CH3COOH D�� HCl
��2���ڲ�����м�������KCl��____________________�����˵õ�K2Cr2O7���塣
��3��ij������akg ������ۣ���Cr2O3 40%����K2Cr2O7�����յõ���Ʒ b kg������Ϊ_____________________________��100%�����м���ʽ����
��4������ԭ���ɳ�ȥ��ˮ�е�Cr2O72-��ȡ��Cr2O72-��ģ��ˮ���ֱ��ڲ�ͬPH������,��ÿ��ˮ���з� ���һ������FeSO4��NaHSO3�����裬��ַ�Ӧ,Ȼ��μ�Ca(OH)2����Һ,���ó������ⶨ��ȥ����,ʵ������ͼ��ʾ��

����������������NaHSO3ʹCr2O72-��ԭ��ΪCr3+,��д��NaHSO3��Cr2O72-��Ӧ�����ӷ���ʽ:____________________________________��
�� pH>8ʱ�������ζ�+6��Cr��ȥ��Ч�������½�,���ܵ�ԭ����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪2-��ϩ��˳���������칹�壬���������¿������崦��ƽ�⣬

 (g)+H2(g)��CH3CH2CH2CH3(g) ��H=-118.9kJ/mol��
(g)+H2(g)��CH3CH2CH2CH3(g) ��H=-118.9kJ/mol��
����˵����ȷ����
A. ˳-2-��ϩ�ȷ�-2-��ϩ�ȶ�
B. ��ѹ�ͽ���������ƽ��������˳-2- ��ϩ��Ӧ�����ƶ�
C. .˳-2-��ϩ��ȼ���ȱȷ�-2-��ϩС
D. ��-2-��ϩ�⻯���Ȼ�ѧ����ʽΪ (g)+H2(g)��CH3CH2CH2CH3(g) ��H=-114.7kJ/mol
(g)+H2(g)��CH3CH2CH2CH3(g) ��H=-114.7kJ/mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com