
����Ŀ����1��CO2���������壬����NaOH��Һ���յõ�Na2CO3��NaHCO3��Na2CO3�׳ƴ����֪25��ʱ��CO32����һ��ˮ���ƽ�ⳣ��Kh=2��10-4mol/L������Һ��c(HCO3-)��c(CO32��)=20�� 1 ʱ����Һ��pH=______��
��2��Ϊ�˳�ȥ��������Ag2S���ɲ������·�������һ�����Ƶ������з���ʳ����Һ������������ʳ����Һ��ʹ���������Ӵ������γ�ԭ��أ���һ��ʱ�䣬���������Ϊ����ɫ�����ŵ�����������ζ���۲쵽��������ɫ��״�������ɣ���ԭ��ص�������ӦΪ______________________________������ͳ�������ζ�γɵ�ԭ�������ӷ���ʽ��ʾ��________________________________________��

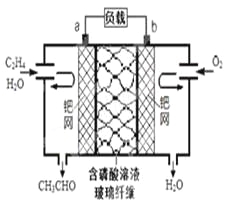
��3��25 ������0.10 mol��L��1H2S��Һ�У�ͨ��HCl��������NaOH�����Ե�����ҺpH����ҺpH��c(S2��)��ϵ��ͼ(������Һ����ı仯��H2S�Ļӷ�)��
��pH��11ʱ����Һ�е�c(H2S)��c(HS��)��________mol��L��1��
��ij��Һ��0.040 mol��L��1M2����0.10 mol��L��1H2S������ҺpH��________ʱ��Mn2����ʼ������[��֪��Ksp(MS)��5.6��10��17]
��4��Na2S2O3��Һ����Ϊ��Һ�ⶨ���ʵ���ɡ�
I.ȡ3.92 gij�����������������ϡ���ᣬ�����Ƴ�100.0 mL��Һ��
II.ȡ10.00 mL������Һ����������KI��Һ���μӼ���ָʾ����
III.��0.2000 mol L-1��Na2S2O3����Һ�ζ����ظ�2��3�Σ�ƽ�����ı�Һ20.00mL��
��֪��I2+2S2O32-= S4O62-+2I-����
�ٲ���II ����ָʾ��������Ϊ____________���жϴﵽ�ζ��յ�IJ���������___________________��
�ڸ�����������Ļ�ѧʽΪ______________��
��5�������£���20 mL 0.2 mol /L H2A��Һ�еμ�0.2 mol /L NaOH��Һ���й��������ʵ����仯����ͼ�����������ߴ�������A2����H2A��HA��Ũ�ȱ仯�����ߣ�����ͼʾ����V(NaOH)��20 mLʱ����Һ��Na����HA���� A2���� H2A������Ũ�ȴ�С��ϵ��__________________________________����Һ��_______�ԡ�

���𰸡� 9 Ag2S +2e- = 2Ag + S2- 2Al3+ + 3S2- + 6H2O = 2Al(OH)3��+3H2S�� 0.0987 3 ������Һ �������һ�α�Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ Fe5O7 Na+>HA->A2->H2A ����
����������1��CO2���������壬����NaOH��Һ���յõ�Na2CO3��NaHCO3��Na2CO3�׳ƴ����֪25��ʱ��CO32����һ��ˮ���ƽ�ⳣ��Kh=2��10-4mol/L������Һ��c(HCO3-)��c(CO32��)=20��1 ʱ����Kh=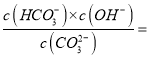 2��10-4mol/L���������
2��10-4mol/L���������![]() mol/L������
mol/L������![]() mol/L����Һ��pH=9��
mol/L����Һ��pH=9��
��2��Ϊ�˳�ȥ��������Ag2S���ɲ������·�������һ�����Ƶ������з���ʳ����Һ������������ʳ����Һ��ʹ���������Ӵ������γ�ԭ��أ���һ��ʱ�䣬���������Ϊ����ɫ�����ŵ�����������ζ���۲쵽��������ɫ��״�������ɣ���ԭ��ص�������ӦΪAg2S +2e- = 2Ag + S2-����������ζ�γɵ�ԭ����Al3+ ��S2-����˫ˮ�ⷴӦ�����г�������ζ�����⣬���ӷ���ʽΪ2Al3+ + 3S2- + 6H2O = 2Al(OH)3��+3H2S����
��3������ͼ��֪��pH��11ʱ��c(S2-)��1.3![]() mol/L���������غ�ɵ�c(H2S)��c(HS��)+ c(S2-)��0.10mol��L��1��������Һ�е�c(H2S)��c(HS��)��0.10mol��L��1-1.3
mol/L���������غ�ɵ�c(H2S)��c(HS��)+ c(S2-)��0.10mol��L��1��������Һ�е�c(H2S)��c(HS��)��0.10mol��L��1-1.3![]() mol/L =0.0987mol��L��1��
mol/L =0.0987mol��L��1��
��ij��Һ��0.040 mol��L��1M2����0.10 mol��L��1H2S����Mn2����ʼ����ʱ��c(S2-)=![]() mol/L����ͼ���е���Ϣ��֪����ʱ��ҺpH��3��
mol/L����ͼ���е���Ϣ��֪����ʱ��ҺpH��3��
��4���ٲ���II ����ָʾ���ǵ�����Һ���жϴﵽ�ζ��յ�IJ��������������������һ�α�Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
���������֪��3.92 gij������������������ϡ���������������������������������KI��Fe3+��I-����ΪI2��Ȼ���ټ���Na2S2O3����Һ��I2��ԭΪI-�����ݵ���ת�Ƶõ���ϵʽ2 Fe3+~ I2~2S2O32������10.00 mL������Һ��n(Fe3+)=n(S2O32)= 20.00![]() 0.2000 mol L-1=0.004000mol����100.0 mL��Һ��n(Fe3+)=0.04000mol�������3.92 gij������������n(Fe2O3)=0.02000mol��n(FeO)=
0.2000 mol L-1=0.004000mol����100.0 mL��Һ��n(Fe3+)=0.04000mol�������3.92 gij������������n(Fe2O3)=0.02000mol��n(FeO)= ![]() ��n(Fe2O3):n(FeO)=2:1�����Ը�����������Ļ�ѧʽΪFe5O7��
��n(Fe2O3):n(FeO)=2:1�����Ը�����������Ļ�ѧʽΪFe5O7��
��5����ͼ���֪��I��II��III�����߷ֱ��������H2A ��HA����A2��Ũ�ȱ仯�����ߣ���V(NaOH)��20 mLʱ��H2A��NaOHǡ�÷�Ӧ����NaHA����ͼ���֪��NaHA��Һ��c(A2)->c(H2A)��˵���ĵ���̶ȴ�����ˮ��̶ȣ���Һ����������ˣ�Na����HA


 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Fe(OH)3 ����� MgCl2 ��Һ��ͬ���е�������
A.������ɫ������Һ
B.��ɢ�����Ӷ���ͨ����ֽ
C.�������������������
D.���ж����ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����������ȩ����Ƴ�����ͼ��ʾ��ȼ�ϵ�أ������Ʊ���ȩ��ͬʱ��õ��ܣ����ܷ�ӦΪ��2CH2��CH2+ O2��2CH3CHO�������й�˵����ȷ����
A. �õ��Ϊ�ɳ����
B. ÿ��0.1mol O2��Ӧ����Ǩ��H+ 0.4mol
C. ������ӦʽΪ��CH2��CH2��2e-+ 2OH-��CH3CHO + H2O
D. �����ƶ����缫a��������Һ���缫b
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ClO2 ��һ�ָ�Ч��ȫ��ɱ�������������Ȼ��Ƶ�ⷨ����ClO2�Ĺ���ԭ��ʾ��ͼ����ͼ, �������ڵ������ClO2������˵����ȷ����

A. a������������b����������
B. �Ȼ��Ƶ�����ÿ����2 mol a���壬ת��2mol e-
C. ClO2�������������ĵ缫��ӦʽΪ��ClO3- + 2H+ + e-![]() ClO2�� + H2O
ClO2�� + H2O
D. Ϊʹa��b����ǡ����ȫ��Ӧ��������ÿ����1molClO2��Ҫ����44.8Lb���壨����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��һ���¶��£�Ksp[Mg3��PO4��2]��6.0��10��29��Ksp[Ca3��PO4��2]��6.0��10��26����Ũ�Ⱦ�Ϊ0.20mol��L��1��MgCl2��CaCl2�����Һ����μ���Na3PO4��������________�������ѧʽ�����������Һ����һ�ֽ��������ӳ�����ȫ��Ũ��С��10��5mol��L��1��ʱ����Һ�е���һ�ֽ��������ӵ����ʵ���Ũ��Ϊ________��
��2������ʯ����Ҫ�ɷ�BaCO3����Ca2����Mg2����Fe3�������ʣ���ʵ�������ö���ʯ�Ʊ�BaCl2��2H2O���������£�

�ٶ���ʯ�������ȡǰ������ĥ��Ŀ����________��
�ڼ���NH3��H2O����pH��8�ɳ�ȥ________�������ӷ��ţ����������к�________���ѧʽ��������H2C2O4ʱӦ���������ԭ����________��
Ca2�� | Mg2�� | Fe3�� | |
��ʼ����ʱ��pH | 11.9 | 9.1 | 1.9 |
��ȫ����ʱ��pH | 13.9 | 11.1 | 3.7 |
��֪��Ksp��BaC2O4����1.6��10��7��Ksp��CaC2O4����2.3��10��9��
��3����֪25��ʱ��CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����100mL�������µ�CaSO4������Һ�м���400mL 0.01mol��L��1 Na2SO4��Һ������������ȷ����___������ĸ����

A����Һ������CaSO4������������Һ��c(SO42��)��ԭ���Ĵ�
B����Һ��������������Һ��c��Ca2������c(SO42��)����С
C����Һ������CaSO4��������Һ��c��Ca2������c(SO42��)����С
D����Һ��������������������Һ��c(SO42��)��ԭ���Ĵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ijͬѧΪ̽������KMnO4��Һ��H2C2O4�����ᣬ��Ԫ���ᣩ��Һ�ķ�Ӧ���̣���������ʵ�顣������������⣺
��д������KMnO4��Һ��H2C2O4�����ӷ���ʽ___________________________________��
������100mL0.0400mol��L-1��H2C2O4��Һ�����õ�������ƽ��ҩ�ס��ձ�����Ͳ���������������⣬�������õ��IJ���������_______________________________________��
�۽�KMnO4��Һ��ε���һ�����������H2C2O4��Һ�У��¶���ͬ����������¼���������£�
����KMnO4��Һ�Ĵ��� | KMnO4��Һ��ɫ��ȥ�����ʱ�� |
�ȵ����1�� | 60s |
��ɫ���ٵ����2�� | 15s |
��ɫ���ٵ����3�� | 3s |
��ɫ���ٵ����4�� | 1s |
�����KMnO4��Һ��ɫʱ��仯�Ŀ���ԭ��___________________________________��
��2��![]() ��
��![]() ����Һ�п��ת���������£���ʼŨ��Ϊ1.0 mol��L-1��Na2CrO4��Һ��
����Һ�п��ת���������£���ʼŨ��Ϊ1.0 mol��L-1��Na2CrO4��Һ��![]() ��c(H+)�ı仯��ͼ��ʾ
��c(H+)�ı仯��ͼ��ʾ

�������ӷ���ʽ��ʾ��Һ��![]() ��
��![]() ��ת����Ӧ_________��
��ת����Ӧ_________��
����ͼ��֪����Һ���Լ�С�� ![]() ��ƽ��ת����_________���������С�����䡱����
��ƽ��ת����_________���������С�����䡱����
�������¶ȣ���Һ��![]() ��ƽ��ת���ʼ�С����÷�Ӧ�Ħ�H_________0������ڡ���С�ڡ����ڡ�����
��ƽ��ת���ʼ�С����÷�Ӧ�Ħ�H_________0������ڡ���С�ڡ����ڡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ��������ʵ�飺��һ�����ڵ���KI��Һ�У���������NaClO��Һ������������ϡ���ᣬ��Һ�����������ڶ�������������ɫ��Һ�У��μ�������Na2SO3��Һ����ɫ����ʧ�������йظ�ͬѧ��ʵ��ԭ���Ľ��ͺ����ý��۵���������ȷ����(����)
A. �����ԣ�ClO����I2��SO42��
B. ��ɫ��ʧ��ԭ����Na2SO3��Һ����Ư����
C. ����KI��Һ��������ΪI����ClO������ΪI2��I2ʹ���۱���
D. ����Na2SO3��Һ������ˮ�У���ˮ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ������������������������������������Һ���������������ͼ����ǣ�
A����������Һ�м�������������Һ
B����̼��������Һ�м����Ȼ���
C�������ᡢ����þ���������Ļ����Һ����μ�������������Һֱ������
D�����Ȼ�����Һ�м����������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯�������ճ�����������Ӧ�ù㷺�IJ��ϡ���ش�����������
��1����̬��ԭ�ӵļ۵��ӹ������ʽΪ_____________________ ��
��2����Ԫ�س�����������Fe2+��Fe3+���ȶ���Fe2+____Fe3+(������������������С����)��ԭ����______________________ ��
��3�������������ܴ�����ƽ���NH4ClO4�ķֽ⣬NH4+�ĽṹʽΪ_________________(�����λ��)���ռ乹��Ϊ_______________�����е�ԭ�ӵ��ӻ���ʽΪ_______________����ClO4-��Ϊ�ȵ�����ķ��ӻ�������______(��дһ��)��
��4��ij�����������������ᄃ����ͼ��ʾ������A��B������ɡ������������Fe2+��Fe3+��O3-�ĸ�����Ϊ___________(�����������)��
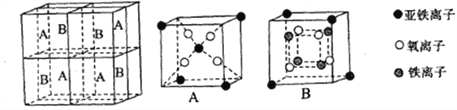
��5��������������������ͬ�������壬����ͼ��ʾ��

��-Fe�����һ�������������е���ԭ����Ϊ________����-Fe����-Fe���־�������ԭ�ӵ���λ��֮��Ϊ_________��
��֪��-Fe������ܶ�Ϊdg/cm3��NA��ʾ����٤����������ֵ����Feԭ�Ӱ뾶Ϊ_______Pm(�б���ʽ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com