
 2CaO+O2��
2CaO+O2��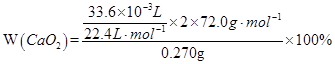 =80.0��
=80.0��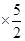 =0.00155mol
=0.00155mol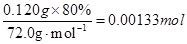
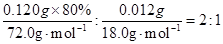
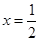
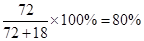 ����Դ:
����Դ: CaO+O2����������������������δ��ƽ���¡�������������δ������ʾ��Ϣ���¡������������ڶ�д��ˮ�ķ���ʽ���¡�������������δͨ��ȫ�����������CaO2��xH2Oд���ж�ֵ��ˮ�����ʧ�ڣ�2���ʣ�������Ϣ���У�O2�ڱ�״���µ��������������ʵ���Ϊ33.6mL��22400mL��mol-1=1.50��10-3mol��
CaO+O2����������������������δ��ƽ���¡�������������δ������ʾ��Ϣ���¡������������ڶ�д��ˮ�ķ���ʽ���¡�������������δͨ��ȫ�����������CaO2��xH2Oд���ж�ֵ��ˮ�����ʧ�ڣ�2���ʣ�������Ϣ���У�O2�ڱ�״���µ��������������ʵ���Ϊ33.6mL��22400mL��mol-1=1.50��10-3mol��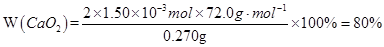
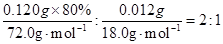



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH4CuSO3����Ԫ�ر������� | B���̼�����ζ�������Ƕ���������� |
| C���÷�Ӧ��NH4CuSO3�������������ǻ�ԭ�� | D���÷�Ӧ������ȱ���Ϊ�����ֱ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2:3 | B��2:1 | C��1:2 | D��3:2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ۢݢ� | B���ڢ� | C���ڢܢ� | D���٢ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��I- > Fe2+ > H2SO3 > NO | B��H2SO3 > I- > Fe2+ > NO |
| C��Fe2+ > I-> H2SO3 > NO | D��NO >Fe2+ > H2SO3 > I- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
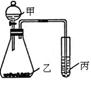
| ʵ���Լ� | ʵ����� | ||
| �� | �� | �� | |
| �� | ������� | �� | �����ԣ�KMnO4��Cl2��Br2 |
 ��1��ͼ�Т�Ϊ ����Ϊ ��
��1��ͼ�Т�Ϊ ����Ϊ �� ��2�������������ʵ��װ�������һ��ʵ�飬����ѡ����Լ������е������Լ�����ʵ������ó���ʵ����������±���
��2�������������ʵ��װ�������һ��ʵ�飬����ѡ����Լ������е������Լ�����ʵ������ó���ʵ����������±���| �Լ��� | �Լ��� | �Լ��� | �������� | ʵ����� |
| | | | | ���ԣ����̼�� |
| | | ʯ����Һ | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 X+H2 ��Y+NaOH��G+W+H2O
X+H2 ��Y+NaOH��G+W+H2O | A��W��G��Z��Y��X | B��G��Y��W��Z��X |
| C��G��Y��Z��W��X | D��Z��X��G��Y��W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com