
����Ŀ�����й������ʽṹ�������У������������
��CH3COOH������̼ԭ�ӵ��ӻ�������sp2��sp3����
��Ԫ��Geλ�����ڱ���������IVA�壬��������Ų�ʽΪ[Ar]4s24p2������P��
�۷Ǽ��Է����������и߶ȶԳ��ԣ���BF3��H2O2��CO2�����ķ���
�ܱ��д��ڼ��Թ��ۼ���������ֻ�ѧ��������
��Cu(OH)2��һ����ɫ��״���������������ᡢ��ˮ��Ҳ����������������Һ��
������̬��HgCl2�����磬HgCl2ϡ��Һ�����ĵ�������˵����̬HgCl2�ǹ��ۻ����Ϊ�ǵ����
�߰�ˮ�д�NH3��H2O��������á�����������ʾ����ϳ�NH3��H2O���ӣ����ݰ�ˮ�����ʿ�֪NH3��H2O�Ľṹʽ�ɼ�Ϊ��
A.4��B.5��C.6��D.7��
���𰸡�A
��������
�ټ���̼ԭ�ӣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ���=4+0=4������Ϊsp3�ӻ���-COOH�У�̼ԭ�Ӽ۲���ӶԸ���=3+0=3���γ�3���Ҽ����¶Ե��ӣ��ӻ���ʽΪsp2�ӻ�������ȷ��
��Ge�Ǣ�A�������Ԫ�أ����������Ų�ʽΪ[Ar]3d104s24p2������P��Ԫ�أ��ʴ���
��H2O2Ϊ���Է��ӣ���BF3��CO2Ϊ�Ǽ��Է��ӣ��ṹ�Գƣ��ʴ���
��������ǻ�ѧ�����ʴ���
��������ͭ�ܺ��ᷢ���кͷ�Ӧ�������ᣬ������ͭҲ�ܺͰ�ˮ��Ӧ������������ӣ�������������ʽ�Σ���ˮ��Һ���ܵ���������ӣ�������ͭ����������������Һ�У�����ȷ��
��HgCl2��ϡ��Һ�����ĵ���������˵����������ʣ��ʴ���
�����Ӧ�γ���X��H-Y��ʽ���У�X��Y������N��O��FԪ��֮һ�������������ֿ��ܣ�(1)H3N��H-O-H��(2)H2N-H��OH2������һˮ�ϰ��ɵ����NH4+��OH-������(1)�ṹ�Ǻ����ģ�����ȷ��
������Тڢۢܢ�4��ʴ�ѡA��


 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)����CO��H2����CH3OCH3������;����
CO(g)+H2(g)�TCH3OH(g) ��H1��-91kJmol-1 ƽ�ⳣ��K1
2CH3OH(g)�TCH3OCH3(g)+H2O ��H2��-24kJmol-1 ƽ�ⳣ��K2
CO(g)+H2O(g)�TCO2(g)+H2(g) ��H3��-41kJmol-1 ƽ�ⳣ��K3
�¹��յ��ܷ�ӦʽΪ3CO(g)+3H2(g)�TCH3OCH3(g)+CO2(g)��÷�Ӧ����H��_____����ѧƽ�ⳣ��ΪK��__(��K1��K2��K3�Ĵ���ʽ��ʾ)��
(2)һ������������A������B��Ӧ��������C����Ӧ�����з�Ӧ�����������Ũ����ʱ��仯��������ͼ���˷�Ӧ�ڴﵽƽ��ʱ��A��ת����Ϊ__��

(3)�о������ж�������ŷŶԸ��ƻ�������������ʮ����Ҫ�����塣����I2O5����CO��Ⱦ�ķ�ӦΪ��5CO(g)+I2O5(s)5CO2(g)+I2(s) ��H����ͬ�¶��£���װ������I2O5�����2L�����ܱ�������ͨ��4molCO�����CO2�����������(CO2)��ʱ��t�仯������ͼ��

��ش�
�ٸ÷�Ӧ����__��Ӧ(��������������������)��
�ڴӷ�Ӧ��ʼ��a��ʱ�ķ�Ӧ����Ϊv(CO)��___��b��ʱ��ѧƽ�ⳣ��Kb��__��
������˵������ȷ����____(����ĸ���)��
a�������������ܶȲ��䣬������Ӧ�ﵽƽ��״̬
b�������¶��£�c��ʱ��ϵ�л�������ѹǿ���
c������d�����ϵѹǿ��CO��ת���ʲ���
d��b���d��Ļ�ѧƽ�ⳣ����Kb��Kd
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ҫ�ɷֵĽṹ��ʽ��ͼ��
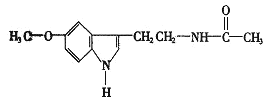
���ж�����Ҫ�ɷֵ����۴�����ǣ� ��
A.�����ʽΪC13H16N2O2
B.��ˮ����������
C.������ˮ�����ӳɷ�Ӧ
D.��Ӫ���ɷּ�����������������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ����
A.��FeCl3��Һ��ʴӡˢ��·����ͭ����2Fe3++Cu=2Fe2++Cu2+
B.Fe��ϡ���ᷴӦ��2Fe+6H+=2Fe3++3H2��
C.����������Һ�����ᷴӦ��Ba2++![]() +OH-+H+=H2O+BaSO4��
+OH-+H+=H2O+BaSO4��
D.��С�մ���Һ�е�����![]() +2CH3COOH��CO2��+H2O+2CH3COO��
+2CH3COOH��CO2��+H2O+2CH3COO��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����Ԫ�ص�ԭ�������Ĵ�С˳��ΪC>A>B>D>E��A��Cͬ���ڣ�B��Cͬ���壻A��B�γ����ӻ�����A2B��A2B���������ӵĵ�������ͬ�����������Ϊ30��D��E���γ�4��10���ӷ��ӡ��Իش��������⣺
��1��д������Ԫ�ص����ơ�
A________��B________��C________��D__________��E________��
��2���õ���ʽ��ʾ���ӻ�����A2B���γɹ�����__________________________________��
��3��д��DԪ���γɵĵ��ʵĽṹʽ____________________________�����к�____��������______��������
��4��д���������ʵĵ���ʽ��E��B�γɵĻ�����(��дһ��)________________��A��B��E�γɵĻ�����________________��D��E�γɵĻ�����____________��
��5��A��B��Ԫ����ɵĻ�����A2B2����________(ѡ������������������)��������ڵĻ�ѧ����____________��д��A2B2��ˮ��Ӧ�Ļ�ѧ����ʽ��_________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com