
����Ŀ���Ϸ���ij���л�ѧ�����Կγ�ѧϰС���������⣨Fe2O3������ϵ��ʵ�飬����֮��Ĺ�ϵͼ���¡�
������ѧ֪ʶ�ش��������⣺
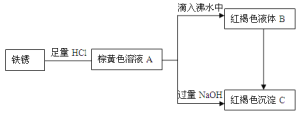
IHCl������
ʵ�������ܶ�Ϊ1.18g/mL����������Ϊ36.5%Ũ��������480mL0.1mol/L��������Һ��
��1������480mL0.1mol/L��������Һ��ҪŨ��������Ϊ___mL��(����2λ��Ч����)
��2�������ձ�������������Ͳ����ͷ�ιܺ��Լ�ƿ����Ҫ��������___��
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죿(�ƫ�͡���ƫ�ߡ�)
δϴ���ձ�___������ʱ���ӿ���____��
II̽��ʵ��
��1��д����A���뵽��ˮ���Ʊ�B�Ļ�ѧ����ʽ��___
��2������˵����ȷ����___������ţ���
��Bת����C�����˻�ѧ��Ӧ
����A�Ʊ�B������Խ��Խ��
������B���ж����ЧӦ
�ܰ�B��C��ɵĻ������ˣ���Һ����ɫ��
���������̶����漰��������ԭ��Ӧ
��3������ɫ����C�м���NaClO��NaOH�����Һ������һ�ָ�Чɱ����ˮ��Na2FeO4����֪ÿ����0.2mol��Na2FeO4����0.3molNaClO����÷�Ӧ�Ļ�ԭ����Ϊ___��
���𰸡�4.2 500mL����ƿ ƫ�� ƫ�� FeCl3+3H2O![]() Fe(OH)3�����壩+3HCl �ۢ� NaCl
Fe(OH)3�����壩+3HCl �ۢ� NaCl
��������
I.��1��������������Һ�����ѡ����ʹ�������ƿ������![]() ����Ũ��������ʵ���Ũ�ȣ���������Һϡ���������ʵ����ʵ������������ҪŨ����������
����Ũ��������ʵ���Ũ�ȣ���������Һϡ���������ʵ����ʵ������������ҪŨ����������
��2��������Ũ��Һ����һ�����ʵ���Ũ�ȵ�ϡ��Һ��һ�㲽��ȷ��ʵ������������
��3���������������ʵ����ʵ�������Һ�����Ӱ�죬����![]() ������������
������������
II. ���⣨Fe2O3�������������ᷴӦ���ɵ�AΪ�Ȼ������Ȼ��������ˮ�л���B�����������壬�Ȼ������������Ʒ�Ӧ������C��������������
��1�����Ȼ��������ˮ���Ʊ����������������õ����Ȼ�����ˮ�⣻
��2����Bת����CΪ����ľ۳���
�ڽ��Ȼ��������ˮ���Ʊ�������������ʱ������ʱ�������ʹ����۳������ܳ�ʱ����ȣ�
�۽�����ж����ЧӦ��
����������������ͨ����ֽ������������������ͨ����ֽ��
�ݸ���Ԫ�ػ��ϼ��Ƿ�仯�ж��Ƿ����������ԭ��Ӧ��
��3��������Ϣ��֪Fe(OH)3ʧ���ӱ���������Na2FeO4��NaClO���������ԣ����������������õ��ӣ���ClԪ�صõ��Ӻ�Ļ��ϼ�Ϊx�����ݵ�ʧ�����غ����x��ֵ���Ӷ�ȷ����ԭ���
I.��1������480mL0.1mol/L��������Һ��Ӧѡ��500mL����ƿ���ܶ�Ϊ1.18g/mL����������Ϊ36.5%Ũ�������ʵ���Ũ��![]() ������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ������䣬�ɵ�11.8mol/L��V=0.5L��0.1mol/L�����V=4.2mL��
������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ������䣬�ɵ�11.8mol/L��V=0.5L��0.1mol/L�����V=4.2mL��
�ʴ�Ϊ��4.2��
��2����Ũ��������ϡ�����ʵ�鲽����������㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ����ʵ�鲽���֪��ʵ���������������ձ�������������Ͳ����ͷ�ιܺ��Լ�ƿ����Ҫ��������500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
��3��δϴ���ձ��������ʻ���ʧ��ʹ���Ƶ���ҺŨ��ƫ�ͣ�����ʱ���ӿ��ߣ���ʹ���������ˮ�����ƫС����ʹ���Ƶ���Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ͣ�ƫ�ߣ�
II. ���⣨Fe2O3�������������ᷴӦ���ɵ�AΪ�Ȼ������Ȼ��������ˮ�л���B�����������壬�Ȼ������������Ʒ�Ӧ������C��������������
��1�����Ȼ��������ˮ���Ʊ�������������Ļ�ѧ����ʽ�ǣ�FeCl3+3H2O![]() Fe(OH)3�����壩+3HCl��
Fe(OH)3�����壩+3HCl��
�ʴ�Ϊ��FeCl3+3H2O![]() Fe(OH)3�����壩+3HCl��
Fe(OH)3�����壩+3HCl��
��2����Bת����CΪ����ľ۳�������ľ۳��������仯���̣��ʢٴ���
�ڽ��Ȼ��������ˮ���Ʊ�������������ʱ������ʱ�������ʹ����۳������ܳ�ʱ����ȣ��ʢڴ���
����������������ж����ЧӦ���ʢ���ȷ��
�ܰ�����������������������������ˣ���������������ͨ����ֽ����Һ�Ǻ��ɫ���ʢܴ���
��������Ӧ����û��Ԫ�ػ��ϼ۵ı仯����������ԭ��Ӧ���ʢ���ȷ��
�����������ۢ���ȷ��
�ʴ�Ϊ���ۢݣ�
��3��������Ϣ��֪Fe(OH)3ʧ���ӱ���������Na2FeO4��NaClO���������ԣ����������������õ��ӣ���ClԪ�صõ��Ӻ�Ļ��ϼ�Ϊx�����ݵ�ʧ�����غ�ɵ�![]() ����ã�x=-1����÷�Ӧ�Ļ�ԭ����ΪNaCl��
����ã�x=-1����÷�Ӧ�Ļ�ԭ����ΪNaCl��
�ʴ�Ϊ��NaCl��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ˮ�е�NO3�����ཡ�������Σ����Ϊ�˽�������ˮ��NO3��Ũ�ȣ������ڼ��������������۽�NO3��ԭΪN2���仯ѧ����ʽΪ��
10Al��6NaNO3��4NaOH��10NaAlO2��3N2����2H2O����ش��������⣺
(1)������Ӧ�У���������Ԫ��_________����ԭ������________��
(2)�á�˫���ŷ�����ʾ��Ӧ�е���ת�Ƶķ������Ŀ��_______________��
10Al��6NaNO3��4NaOH��10NaAlO2��3N2����2H2O
(3)��Ӧ��ÿ���ɱ����22.4L N2��ת��_______mol���ӡ�
(4)����ƽ�������ӷ���ʽ��
____Fe2++____H+ +____NO3��____Fe3+ +____N2O��+____H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������ϩ�����������ʵ�װ��ͼ����ش�

(1)д��Բ����ƿ�з�Ӧ�ķ���ʽ______________________________��
(2)��ƿ�л��Һ��ڣ�������ijЩ���������壬д��������������Ļ�ѧ����ʽ��____________________________________________��
(3)Ϊ�˼�����ϩ�����ɼ���ϩ�IJ������ԣ��Թ���Ӧʢ��_________������Ϊ___________��
(4)ϴƿ��ʢ�ŵ��Լ�Ϊ____________________������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ����ѡ���װ�û�����(�г�װ������ȥ)��ȷ���ǣ� ��
A | B | C | D | |
ʵ�� | ��ȡ����������CO2���� | ��CCl4��ȡ��ˮ�е�Br2 | ��ȥCO2��������HCl | ����NaCl������Һ�Ʊ�NaCl���� |
װ�û����� |
|
|
|
|
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪Һ����������ˮ���ơ�T ��ʱ��NH3+NH3![]() NH4 ++NH2-��NH4+��ƽ��Ũ��Ϊ1��10-15 mol��L-1��������˵������ȷ����( )
NH4 ++NH2-��NH4+��ƽ��Ũ��Ϊ1��10-15 mol��L-1��������˵������ȷ����( )
A. �ڴ��¶���Һ�������ӻ�Ϊ1��10-14
B. ��Һ���з�������ƣ�������NaNH2
C. �����£���Һ���м���NH4Cl����ʹҺ�������ӻ���С
D. ���£���ʹҺ������ƽ�������ƶ�����c(NH4+)<c(NH2-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ�أ�Se���ǵ�4���ڢ�A��Ԫ�أ������к����������ͻ��������������õİ뵼����ϣ�����������������ڹ������µ����Կ���߽�ǧ����������������ܡ���ش��������⣺
��1����̬Seԭ�Ӽ۵��ӵĹ����ʾʽΪ__��
��2��As��Se��ͬһ����Ԫ�أ�As�ĵ�һ�����ܱ�Se��ԭ����___��
��3�����ڿ�����ȼ��������SeO2�������£�SeO2���ӷ��İ�ɫ���壬�۵�Ϊ340~350�棬315��ʱ��������SeO2������___���壻д��һ����SeO2��Ϊ�ȵ�����������ӵĻ�ѧʽ__��
��4��H2SeO4��H2SO4���ƣ���һ�ֲ��ӷ���ǿ�ᡣSeO42-�Ŀռ乹��Ϊ___������ԭ�ӵ��ӻ���ʽΪ___��
��5������п��һ����Ҫ�İ뵼����ϣ��侧���ṹ��ͼ��ʾ��Znԭ�ӵ���λ��Ϊ___�����þ����ܶ�Ϊ��g��cm-3������п��Ħ������ΪMg��mol-1����NA���������ӵ�������������a���������ı߳���Ϊ___nm��
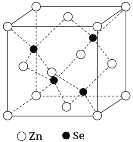
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ӹ����������仯�������������������е����÷����˾�ı䡣
(1)�Ŵ��й��Ĵ���֮һ��ָ����������Ȼ��ʯ�Ƴɵ�,����Ҫ�ɷ���____(����ĸ���)��
a��Fe b��FeO c��Fe3O4 d��Fe2O3
(2)����������Ҫ��ѧ�ɷ�Ϊ��SiO2Լ45%��Fe2O3Լ40%��Al2O3Լ10%��MgOԼ5%���ø÷�����ȡҩ�ø��Ϻ��������Ĺ�����������(���ֲ�����������)��
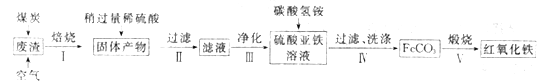
���ڲ�����в������ж����������_____________________��
���ڲ��������У�Ҫ��ȥ������֮һΪAl3+��������ʱKsp[Al(OH)3]=1.0��10-32����ʱ�����Ͻ�Al3+ ������ȫ������Һ��pHΪ_________��(c(Al3+)��1.0��10-5mol/L ��ΪAl3+������ȫ)
�۲�����У�����FeCO3�����ӷ���ʽ��____________________________��
(3)�Ȼ�����Һ��Ϊ��ѧ�Լ��е����������������Ȼ�ͭ���Ȼ����Ļ����Һ�м�������ͭ��ĩ�����������д���ó����Ļ�ѧʽ______________������ƽ���ƶ���ԭ������ϱ�Ҫ�����ӷ���ʽ���Դ������������ͣ�__________________________________________________________��
(4)�ٹ��϶��������ɫȾ����³ʿ���ĺϳɷ������£�

���ֽⷴӦ������ӷ���ʽ��____________________________________________��
����������³ʿ���ϳ�ԭ���ɼ��ʳƷ���Ƿ�CN-���������£�
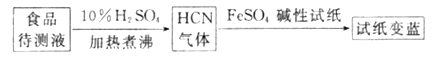
����ֽ������֤��ʳƷ�к���CN-������ͼ��ʱ��ֽ��FeSO4�����ã�____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ϊ��Ҫ�������ϣ��ڻ���������й㷺��Ӧ�á�
��1����̬Asԭ�ӵļ۲���ӵĵ���������ͼ��״Ϊ_____________������ͬ���ڵ�����Ԫ�صĻ�̬ԭ���У���һ����������Ϊ_____________����Ԫ�ط��š���
��2��Na3AsO3�����ڵ����������
��Na+����ɫ��Ӧ�ʻ�ɫ������Ԫ���ܲ�����ɫ��Ӧ����ԭ��Ϊ__________________________��
��Na3AsO3�����������ӵ����幹��Ϊ_____________��д��һ�����以Ϊ�ȵ�����ķ��ӣ�_____________���ѧʽ����
��3��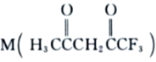 �����ںϳ�Ni2+�����壬M��Cԭ�ӵ��ӻ���ʽΪ___________��������
�����ںϳ�Ni2+�����壬M��Cԭ�ӵ��ӻ���ʽΪ___________��������![]() ������������Ŀ֮��Ϊ___________��
������������Ŀ֮��Ϊ___________��
��4��Ni��Ca����ͬһ���ڣ��Һ����������ӹ�����ͬ��������Ni���۵�ͷе���Ƚ���Ca�ĸߣ���ԭ��Ϊ___________�����־���Ni�ͷǾ���Ni����ɿ��Ŀ�ѧ����Ϊ___________��
��5��ij�����Ͻ�ľ����ṹ����ͼ��ʾ���谢���ӵ�������ֵΪNA����þ�����ܶ���=___________g��cm-3��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ��1mol��L��1��CH3NH2��1mol��L��1��NH2OH��NH2OH+H2O![]() NH3OH++OH�������ּ���Һ����ʼʱ�������Ϊ10mL���ֱ�������Һ�м�ˮ����ϡ�ͣ�����������ͼ��ʾ��V��ʾ��Һ���������pOH=��lgc��OH����������˵����ȷ����
NH3OH++OH�������ּ���Һ����ʼʱ�������Ϊ10mL���ֱ�������Һ�м�ˮ����ϡ�ͣ�����������ͼ��ʾ��V��ʾ��Һ���������pOH=��lgc��OH����������˵����ȷ����

A. NH2OH�ĵ��볣��K��������Ϊ10��8
B. CH3NH3Cl����Һ��ˮ�����ӷ���ʽΪ��CH3NH2+H2O![]() CH3NH3++OH��
CH3NH3++OH��
C. ������Һ��ϡ����lg![]() =4ʱ����Һ��ˮ�ĵ���̶ȣ�NH2OH >CH3NH2
=4ʱ����Һ��ˮ�ĵ���̶ȣ�NH2OH >CH3NH2
D. Ũ����ͬ��CH3NH3Cl��NH3OHCl�Ļ����Һ������Ũ�ȴ�С��ϵ��c��CH3NH3+��<c��NH3OH+��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com