
[���]ÿƬ������������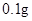 [��Ӧ֢]����ȱ����ƶѪ֢��Ԥ���������á� [�����÷�]����Ԥ���� 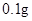 /�գ����������� /�գ�����������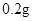 �� ��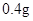 /�ա� /�ա�С������Ԥ���� 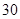 �� ��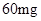 /�գ������� /�գ�������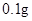 �� ��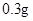 /�� /��[����]�ܹ⡢�ܷ⡢�ڸ��ﴦ���档 |
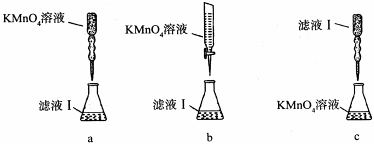
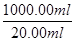 ��0.03 mol��m(Fe2+)��0.03 mol��56 g/mol��1.68 g������Fe2+�������ʣ�
��0.03 mol��m(Fe2+)��0.03 mol��56 g/mol��1.68 g������Fe2+�������ʣ�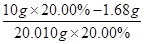 ��16.00%��
��16.00%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A����������ƽ����17.55g�Ȼ��ƾ��� |
| B��̼������Һ�����ڴ����������Լ�ƿ |
| C���ø����pH��ֽ�ⶨ������ˮ��pH |
| D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ�� |
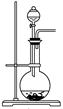
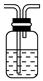
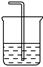
| ���� | O2 | Cl2 | NH3 |
| ��Һ©�����Լ� | | | Ũ��ˮ |
| Բ����ƿ���Լ� | | KMnO4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
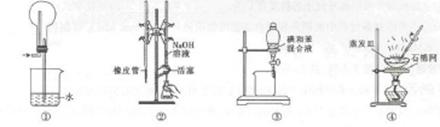
| A��ͼ����ʾװ�ÿ�����HCl���� |
| B��ͼ����ʾװ�ý�������֪Ũ�ȵ�NaOH��Һ�ⶨ����Ũ�ȵ�ʵ�� |
| C��ͼ����ʾװ�ý����ñ���ȡ��ˮ�е��ʵ�飬���ѵ�ı���Һ��©���¿ڷų� |
| D��ͼ����ʾװ������NaCl��Һ���õ�NaCl���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������������Ϊ10%��ZnSO4��Һ����10 g ZnSO4��7H2O�ܽ���90 gˮ�� |
| B�����Ʊ�Fe(OH)3���壬��ʢ�з�ˮ���ձ��еμ�FeCl3������Һ����ʱ����� |
| C��Ϊ����KCl��AlCl3��MgCl2��Һ���ֱ���������Һ�еμ�NaOH��Һ������ |
| D��Ϊ��С�к͵ζ�����ƿ����ϴ������ɺ����ʹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
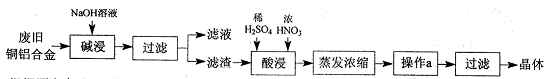
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����25mL��Ͳ��ȡ12.36mL���ᣬ | B����������ƽ��ȡ8.75gʳ�Σ� |
| C������ʽ�ζ�����ȡ23.22mL���������Һ�� | D���ù㷶pH��ֽ���ij��ҺpHΪ3.5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B������ | C����ȡ | D�������ЧӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ͨ��������ú���У���������ͨ��������ˮ�� |
| B����ʹ��ʪ�ĵ���KI��ֽ�����ɫ������ֻ����������������������������̬���� |
| C��Ҫ���ܽ���CCl4�еĵ���������������������Ϊ�����������ȷ������ |
| D��ij��Һ����BaCl2��Һ������������ϡ����İ�ɫ����������Һһ������SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Ʒ�����ȡ���ȴ������CuO |
| B��������Ʒ�����ȡ���ȴ������Cu(NO3)2 |
| C��������Ʒ����NaOH�����ˡ����ȡ���ȴ������CuO |
| D��������Ʒ�����ȡ�����֪��������ˮ�Ȼ�������ˮ���������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com