
��1�������û����ҺΪ���ԣ���a��b=__________������Һ�и������ӵ�Ũ���ɴ�С������˳����__________��
��2�������û��Һ��pH=2����a��b=__________������Һ�и������ӵ�Ũ���ɴ�С������˳����__________��
��������Ϊ������H2O��KW=10-14����t ��ʱKW����25 ��ʱ��KW����Ϊ�¶����ߣ����������ȵģ�ƽ��������Ӧ�����ƶ���KW=c(H+)c(OH-)���
��1��pH=11��NaOH a L��pH=1��H2SO4 b L��Ϻ���Һ�����ԣ���10-2 mol��L-1��a L=10-1 mol��L-1��b L
a��b=10��1
���ݵ���غ�c(Na+)+c(H+)=c(OH-)+2c(![]() )������c(Na+)��c(
)������c(Na+)��c(![]() )��c(H+)=c(OH-)��
)��c(H+)=c(OH-)��
��2��![]() =10-2 mol��L-1
=10-2 mol��L-1
�ɼ���֪a��b=9��2
��aΪ9 L��bΪ2 L����Ӧ��
n(![]() )=
)=![]() ��2 L=0.1 mol
��2 L=0.1 mol
n(H+)=0.01 mol��L-1��(9 L+2 L)=0.11 mol
n(Na+)=0.01 mol��L-1��9 L=0.09 mol
����c(H+)��c(![]() )��c(Na+)��c(OH-)��
)��c(Na+)��c(OH-)��
�𰸣��� 25 ��ʱˮ��KW=1��10-14(С��1��10-13)��ˮ�ĵ��������ȵģ��¶����ߣ�KW����
��1��10��1 c(Na+)��c(![]() )��c(H+)=c(OH-)
)��c(H+)=c(OH-)
��2��9��2 c(H+)��c(![]() )��c(Na+)��c(OH-)
)��c(Na+)��c(OH-)


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| �� |
| ||
| �� |
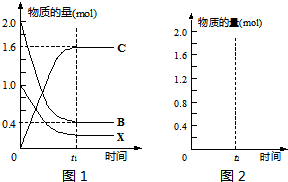
| ���� |
| �� |
| ���� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�꽭��ʡ�߶���ѧ�����п��Ի�ѧ���������� ���ͣ������
(10��)ij�¶�(T��)ʱ�����0.01 mol/L NaOH��Һ��pHΪ11������¶���ˮ��KW��________������¶�________����(����ڡ�����С�ڡ����ڡ�)25�棬��������_________________________________________����������
���¶��£���pH��a��NaOH��ҺVa L��pH��b��H2SO4��ҺVb L��ϣ�ͨ��������д���²�ͬ���ʱ����Һ������ȣ�
(1)�����û����ҺΪ���ԣ���a��12��b��2����Va��Vb��________��
(2)�����û����ҺΪ���ԣ���a��b��12����Va��Vb��___ ��
(3)�����û����Һ��pH��10����a��12��b��2����Va��Vb��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ѡ����
(10��) ij�¶�(t��)ʱ�����0.01 mol/L��NaOH��Һ��pHΪ11������¶���ˮ��Kw��________�����¶�________25��(����ڡ���С�ڡ����ڡ�)����������________________________________________________________________________
________________________________________________________________________.
���¶��£���pH��a��NaOH��ҺVa L��pH��b��H2SO4��ҺVb L��ϣ�ͨ��������д���²�ͬ���ʱ����Һ������ȣ�
(1) ��������ҺΪ���ԣ���a��12��b��2����Va��Vb��____________��
(2) ��������ҺΪ���ԣ���a��b��12����Va��Vb��________��
(3) ��������Һ��pH��10����a��12��b��2����Va��Vb��______________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ�걣���и߶��꼶�ڶ�ѧ������������ѧ�Ծ� ���ͣ������
��11�֣�ij�¶�(t��)ʱ��ˮ�����ӻ�ΪK����1��10��13������¶�(ѡ����ڡ�С�ڻ����)_______25�棬��������_______________________________���������¶���pH��11�Ŀ�������ҺaL��pH��1��ϡ����bL���(���Ϻ���Һ�����С�仯���Բ���)����ͨ��������д���²�ͬ���ʱ������Һ������ȣ�
(1)�����û��ҺΪ���ԣ���a��b��_____������Һ�и������ӵ�Ũ���ɴ�С����˳����_________________��
(2)�����û��Һ��pH��2����a��b��____________������Һ�и������ӵ�Ũ���ɴ�С����˳����_____________ _______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com