
����Ŀ����A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������A��E��������Aԭ�Ӻ���ֻ��һ�����ӣ���֪B��Dԭ������ͬ�ĵ��Ӳ�������B��L���������K���������������E�ڿ�����ȼ��ʱ���ֻ�ɫ���棬E�ĵ����ڼ�������D�ĵ��ʳ�ַ�Ӧ�����Եõ�����ɫ��̬������Ը������������ش�
(1)д��Ԫ��C�����ڱ���λ��________________
(2)E�ĵ����ڼ�������D�ĵ��ʳ�ַ�Ӧ�����Եõ�����ɫ��̬������ĵ���ʽ_________________
(3)C���⻯��������ۺ����ᷴӦ�Ļ�ѧ����ʽ�������������ӵĵ���ʽ__________��__________
(4)B��C��D���⻯����ȶ��Ե��ɴ�С��˳��Ϊ��___________(�⻯��Ļ�ѧʽ)�������ǣ�________________
���𰸡��ڶ����ڵ�VA�� ![]() NH3+HNO3=NH4NO3
NH3+HNO3=NH4NO3 ![]() CH4��NH3��H2O �ǽ�����Խǿ����̬�⻯����ȶ���Խ�ã����ߵķǽ����ԣ�C��N��O�����ȶ��ԣ�CH4��NH3��H2O
CH4��NH3��H2O �ǽ�����Խǿ����̬�⻯����ȶ���Խ�ã����ߵķǽ����ԣ�C��N��O�����ȶ��ԣ�CH4��NH3��H2O
��������
Aԭ�Ӻ���ֻ��һ�����ӣ���AΪH��B��L���������K�����������������Bԭ�ӵĺ����������Ϊ2+4=6��BΪC��E�ڿ�����ȼ��ʱ���ֻ�ɫ���棬��EΪNa��E(Na)�ĵ����ڼ�������D�ĵ��ʳ�ַ�Ӧ�����Եõ�����ɫ��̬�������õ���ɫ��̬������ΪNa2O2��DΪO��C��ԭ��������B(C)��D(O)С����CΪN������������A��B��C��D��E�ֱ�Ϊ��H��C��N��O��Na���ݴ˽��
(1)Ԫ��CΪN����λ��Ԫ�����ڱ��ĵڶ����ڵ�VA�壬�ʴ�Ϊ���ڶ����ڵ�VA�壻
(2)�õ���ɫ����ΪNa2O2�������ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)Ԫ��CΪN�����⻯��ΪNH3������ۺ�����ΪHNO3��NH3��HNO3��Ӧ����NH4NO3���仯ѧ����ʽΪ��NH3+HNO3=NH4NO3��������NH4+�ĵ���ʽΪ��![]() ���ʴ�Ϊ��NH3+HNO3=NH4NO3��
���ʴ�Ϊ��NH3+HNO3=NH4NO3��![]() ��
��
(4)B��C��D�ֱ�Ϊ��C��N��O���ǽ�����Խǿ����̬�⻯����ȶ���Խ�ã����ߵķǽ����ԣ�C��N��O�����ԣ����ߵ��⻯����ȶ��Ե��ɴ�С��˳��Ϊ��CH4��NH3��H2O���ʴ�Ϊ��CH4��NH3��H2O���ǽ�����Խǿ����̬�⻯����ȶ���Խ�ã����ߵķǽ����ԣ�C��N��O�����ȶ��ԣ�CH4��NH3��H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��Q��W��X��Y��Z��Ԫ�����ڱ��е����λ������ͼ��ʾ������ֻ��ZΪ����Ԫ�ء�������˵������ȷ����
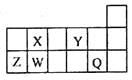
A.W��X����Ԫ������Ȼ���ж�������Ӧ�ĵ���
B.Q��Y�ֱ�����ý���Ԫ���γɵĻ������н������Ӽ�
C.Y��Z�ֱ��γɵļ������У�ǰ�ߵİ뾶�ϴ�
D.X��Z������������Ӧ��ˮ����֮�������������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������Ʊ�ά����B13���м��壬Ҳ���������ܼ���ʵ�����Բ���![]() �ͼ״�Ϊԭ���Ʊ������������һ�ַ������£�
�ͼ״�Ϊԭ���Ʊ������������һ�ַ������£�![]()
![]()
![]() ��
��
������ʵ��й��������±���ʾ��
���� | �� | �״� | ��������� |
�۵�/�� | 5.5 | -97 | 54 |
�е�/�� | 80.1 | 64.3 | 163.5 |
��Է������� | 78 | 32 | 118 |
ˮ���� | ���� | ���� | ���� |
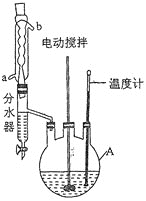
ʵ����̣�����ͼ��Ӧװ���м���27g���ᡢ40g��ˮ�״���100ml����5mlŨ���ᣬ�����¼��Ȼ���������Ӧ��ȫ������״��ͱ������÷�ӦҺ��ˮϴ���л�������10%��̼������Һϴ�ӣ�Ȼ��������ˮϴ�ӣ�����ˮ�Ȼ��ƹ��������ˣ���Һ��ȴ���������壬����ò��������30.1g��
�ش��������⣺
��1������A������Ϊ____________����Ӧ��������ȴˮӦ��________(����a������b��)�ڽ��롣
��2��������״��ͱ��IJ�������Ϊ______________________________��
��3����10%��̼������Һϴ�ӵ�Ŀ����____________________________________��
��4���״�������Ŀ����________________________________________________��Ũ�����������_____________________________________________��
��5����ˮ�����ŵ���__________________________________________(��дһ��)��
��6����ʵ��IJ�����___________(������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
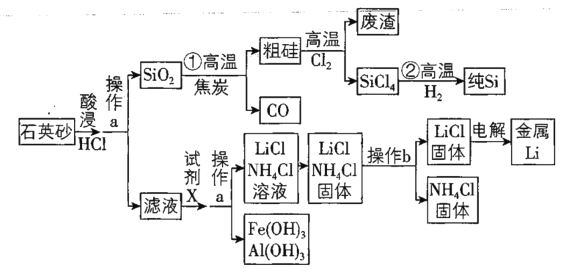
����Ŀ��2018��8��31�գ���Ϊ��˾����AIоƬ����980����־���ҹ��߿Ƽ���ҵ��оƬ���켼�������������ˮƽ�����оƬ�ĺ��������Ǹߴ��ȹ衣��ͼ����ʯӢɰΪԭ��ͬʱ�Ʊ���ͽ���﮵����̡�![]() ʯӢɰ����Ҫ�ɷ�Ϊ
ʯӢɰ����Ҫ�ɷ�Ϊ![]() ����������
����������![]() ��
��![]() ��
��![]()
��֪��LiCl���۵���![]() ���е���
�����![]() ��
��![]() ��
��![]() �ֽ���ȫ�������£�
�ֽ���ȫ�������£�![]() ��
��![]() ��
��
(1)�ֹ��г����и�����SiC����д��![]() ������SiC�ķ�Ӧ����ʽ__________��
������SiC�ķ�Ӧ����ʽ__________��
(2)����aΪ___________���Լ�X��___________��
(3)��֪����bΪ���ȣ���ѡ��������¶���______
![]()
![]()
![]()
![]()
(4)����������Ҫ��Ҫ����LiCl��![]() ��Һ�е���������Ũ����
��Һ�е���������Ũ����![]() ���£�Ӧ������Һ��pHΪ_____����ʱ��Һ��
���£�Ӧ������Һ��pHΪ_____����ʱ��Һ��![]() ��Ũ��Ϊ___________ ��
��Ũ��Ϊ___________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
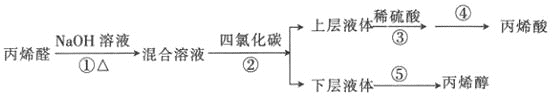
����Ŀ����ϩ���Ǻϳɱ�ϩ��֬��ԭ�ϣ���ϩ�������������͡����ϵȡ�ijС���Ա�ϩȩΪԭ��������ϩ������ϩ���������ͼ��ʾ��
 ��֪��Ӧԭ����
��֪��Ӧԭ����![]()
![]()
�й����ʵ��������������
| ��ϩȩ | ��ϩ�� | ��ϩ�� | ���Ȼ�̼ |
�е�/�� | 53 | 97 | 141 | 77 |
�۵�/�� | -87 | -129 | 13 | -22.8 |
�ܶ�/gcm-3 | 0.84 | 0.85 | 1.02 | 1.58 |
�ܽ���(����) | ������ˮ�� �л��� | ����ˮ�� �л��ܼ� | ����ˮ�� �л��ܼ� | ������ˮ�� �������л��� |
��1����֪���ķе�Ϊ80.1�棬��������ڵ���ȡ�������Ȼ�̼�����ñ�������Ҫԭ�������_________________���ϲ�Һ���Һ©��________���������������������ڵ�����ţ�����
��2���Ӳ���ܵIJ���Һ�п���ȡһ����ˮ���Σ�����________���ѧʽ����
��3������ݲ�����ͼװ�ã���װ����һ�����Դ�����ָ������________������֮���ռ���ϩ�����¶�Ӧ������________���ҡ�
��4����֪��ȩ����н�ǿ��ԭ�ԣ�������ˮ�����Ը��������Һ��������Һ�ȷ�Ӧ��ijͬѧΪ��֤����ϩ���Ʒ�к��б�ϩȩ��������·�����
a.ȡ������ˮ���Թܣ�����������ϩ����Ʒ������Һ��ɫ
b.ȡ�������Ը��������Һ���Թܣ�����������ϩ����Ʒ������Һ��ɫ
c.ȡ����������Һ���Թܣ�����������ϩ����Ʒ����ˮԡ���ȣ�����������
d.ȡ���������ظ������Һ���Թܣ�����������ϩ����Ʒ������Һ�ɳȺ�ɫ�����ɫ
���������У���֤����ϩ���Ʒ�к�������ϩȩ����________������ĸ����
��5������ѡ������ҩƷ���һ����ʵ��֤����ϩ������̼̼˫����________������ѡҩƷ����ϩ����Ʒ�����Ը��������Һ�������ظ������Һ��������Ȼ�̼��Һ��������
��6��Ϊ��֤����ϩ�������ᣬ������·����������ܴﵽʵ��Ŀ�ĵ���________������ĸ����
���� | ���� | Ԥ������ |
A | ȡ���� | ��Һ���ɫ |
B | �����£���10ml0.1molL-1NaOH��Һ��10ml0.1molL-1 | pH>7 |
C | ����ͬ�ĵ����Ƿֱ�ⶨ������Һ�� | ǰ�ߵ��ݽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
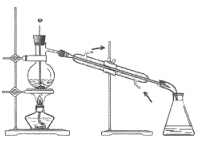
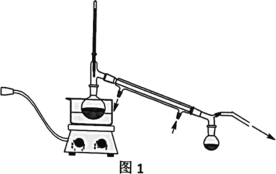
����Ŀ���������Ҷ�ͪ������ҽҩ�м��弰�����߹̻��������ɶ���������ͪ�����Ƶã�������ʵ�������������ѧ����ʽ��װ��ͼ(���Ⱥͼг�װ������ȥ)���£�
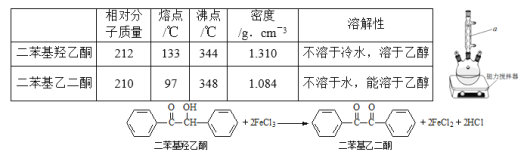
�ڷ�Ӧװ���У�����10ml�����ᡢ5.50gFeCl3���塢10mlˮ���������Ƭ�����������ڣ�ֹͣ���ȣ�������ƽϢ�����2.12g����������ͪ���������Ȼ���������������ͪ��ȫ��Ӧ����Ӧ�������ˮ��У���ȴ���ж������Ҷ�ͪ�ֲ�Ʒ��������70%�Ҵ�ˮ��Һ�ؽᾧ�ᴿ���õ�1.80g��Ʒ��
�ؽᾧ�������£�
�����ܽ������̿��ɫ�����ȹ��� ����ȴ�ᾧ�����ˡ�ϴ�ӡ�����
��ش��������⣺
��1��װ��ͼ������a��������_______________����������______________��
��2���������Ƭ��������________�������Ⱥ���δ�����Ƭ��Ӧ��ȡ����ȷ������____________________________________________________��
��3��ʵ���пɲ��ñ���ɫ���ٷ�Ӧ���̣���ԭ���Ͳ�����ֽ�ϲ�����ͬ��ͨ���۲챡��ɫ��չ����İߵ�(��ʵ�������£�ֻ�ж���������ͪ�Ͷ������Ҷ�ͪ�ܹ������ߵ�)�ж���Ʒ�еijɷ֡���ͼ�ֱ�Ϊ�������������ͪ��Ӧ��ʼ������15min��30min��45min��60minʱ����ëϸ��ȡ��������������ɫ��չ����İߵ㣺
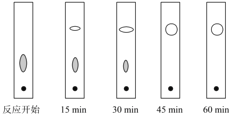
��ʵ�������¼���________�����Ϊ��Ӧ������
A.15min B.30min C.45min D.60min
��4�������ؽᾧ�����У�____________(�������)������ȥ�˲��������ʡ�
��5�����ؽᾧ�����У�����ѡ������ֱ�Ӽ��ȣ�ԭ����_________________________��
��6����ѡ������ķ����ᴿ�������Ҷ�ͪ�ֲ�Ʒ��ԭ����______________________��
��7����ʵ��IJ�����_________%��(����3λ��Ч����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ����(����)
A. �� ��ʾװ�÷���CCl4��I2�Ļ����
��ʾװ�÷���CCl4��I2�Ļ����
B. ��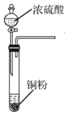 ��ʾװ�û�ȡ����SO2����
��ʾװ�û�ȡ����SO2����
C. ��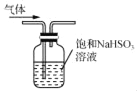 ��ʾװ�ó�ȥCO2�����е�����SO2
��ʾװ�ó�ȥCO2�����е�����SO2
D. �� ��ʾװ������NaCl��Һ���NaCl����
��ʾװ������NaCl��Һ���NaCl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������ૼ�ȩΪԭ���Ʊ�ૼ״���ૼ��ᡣ
���Ʊ�ԭ����
2![]() ��NaOH
��NaOH![]() ��
��![]()
![]() ��HCl
��HCl![]() ��NaCl
��NaCl
��ʵ�鲽��
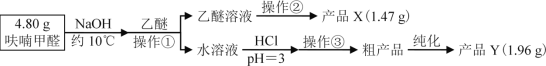
�������Ϣ
ૼ�ȩ | ૼ״� | ૼ��� | ���� | |
�۵�/�� | ��36.5 | ��29 | 133 | ��116.3 |
�е�/�� | 161.7 | 170 | 231 | 34.5 |
ˮ���� | �� | �� | ���� | ���� |
��Է������� | 96 | 98 | 112 | 74 |
����ʵ��װ��
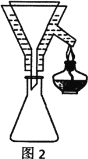
V��������˼�����ش��������⣺
��1������������___
��2�������ڵ�װ����ͼ1��ʾ���ռ���ƷXʱ�¶ȼƵĶ���Ӧ������90�����ң���ԭ����___��
��3������������ˮ��Һ�������������pHΪ2��3��pH��3��������___��������ҺpHʱ��Ӧѡ���ָʾ����__��
��4���ֲ�ƷY����������ͼ2װ�ý����ȹ��ˣ������������ͭ©���м���ˮ��___����װ�á��漰����˳���������ѡ�
A������©��֧��������̾�©����������ֽ���Ž�Һ�ձ��������ȵĴ���Һ
B������̾�©�����Ž�Һ�ձ�������©��֧����������ֽ�������ȵĴ���Һ
C������̾�©����������ֽ������©��֧�����Ž�Һ�ձ��������ȵĴ���Һ
D������̾�©����������ֽ���Ž�Һ�ձ��������ȵĴ���Һ������©��֧��
��5��������30mL��ȡ�����ѣ�����ȡЧ���Ƕ�˼��������4����ȡ��ʽ���������__��
A��30mL��0mL��0mL B��10mL��10mL��10mL
C��15mL��10mL��5 mL D��5mL��10mL��15mL
��6�������ƷY�IJ�����(Y)��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��
��������0.50mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245mL NaOH��Һ��������Ҫ����NaOH����______g��
��2�����±���ѡ�����NaOH��������Ҫ�������ǣ�����ĸ����____��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
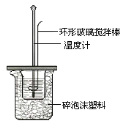
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3kJ/mol����_____��
��2��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
�¶� ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | _____ |
2 | 27.0 | 27.4 | 27.2 | 31.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
��������Ϊ0.50mol/L NaOH��Һ��0.50mol/L������Һ���ܶȶ���1g/cm3���кͺ�������Һ�ı�����c=4.18J/(g����)�����к�����H=___��ȡС�����һλ����
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com