


 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����ˮ
| |||||||||||
B����ˮ
| |||||||||||
C����ˮ
| |||||||||||
D����ˮ
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ | B������ |
| C������ | D������Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
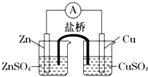
| A��п�ǵ�صĸ�����������ԭ��Ӧ |
| B�������е�������������ͭ��Һ��Ǩ�� |
| C��������п�缫ͨ������������ͭ�缫 |
| D��ͭ�缫�Ϸ����ĵ缫��Ӧ��2H++e-=H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�������ǵ�ȼ������2800 kJ?mol-1����
| ||
B��ȼ�ϵ���н��״�����ת��Ϊ�������Ȼ�ѧ����ʽ�ǣ�CH3OH��g��+
��CH3OH��g����ȼ����Ϊ192.9 kJ?mol-1 | ||
| C��H2��g����ȼ������285.8 kJ?mol-1����2H2O��g���T2H2��g��+O2��g����H=+571.6 kJ?mol-1 | ||
| D����֪H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ?mol-1����H2SO4��Ba��OH��2��Ӧ�ķ�Ӧ�ȡ�H=2����-57.3��kJ?mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ס��ҡ����������졢��Ϊԭ��������������Ķ���������Ԫ�أ��ס�������ͬһ���壬���������촦��ͬһ���ڣ���Ԫ������ϼ�����ͼ۵Ĵ�����Ϊ0�����������������������ˮ����֮�䶼�ܷ�����Ӧ���û�ѧ����ش��������⣺
�ס��ҡ����������졢��Ϊԭ��������������Ķ���������Ԫ�أ��ס�������ͬһ���壬���������촦��ͬһ���ڣ���Ԫ������ϼ�����ͼ۵Ĵ�����Ϊ0�����������������������ˮ����֮�䶼�ܷ�����Ӧ���û�ѧ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H2O+H2O?H3O++OH- |
| B��CO32-+2H2O?H2CO3+2OH- |
| C��Ca��OH��2+2H+?Ca2++2H2O |
| D��Al3++3H2O�TAl��OH��3��+3H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��
| ||
B��
| ||
C��
| ||
D��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ֽⷴӦ | B���û���Ӧ |
| C���ֽⷴӦ | D�����Ϸ�Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com