
��Դ�����ǵ�ǰ����������ٵ�һ���ش���⣬H2��CO��CH3OH������Ҫ����Դ���ʣ����ǵ�ȼ��������Ϊ285.8 kJ/mol��282.5 kJ/mol��726.7 kJ/mol����ش�
(1)��֪CO��H2��һ�������¿��Ժϳɼ״���CO+2H2=CH3OH����H2��CO��Ӧ����CH3OH���Ȼ�ѧ����ʽΪ�� ��
(2)��ͼΪij��ȼ�ϵ�صĹ���ԭ��ʾ��ͼ��a��b��Ϊ���Ե缫��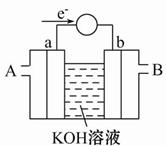
��ʹ��ʱ�������� ��ͨ��(�A����B��)��
�ڼ���ʹ�õġ�ȼ�ϡ��Ǽ״���a���ĵ缫��ӦʽΪ�� ________________
�ۼ���ʹ�õġ�ȼ�ϡ���ˮú��(�ɷ�ΪCO��H2)�����ֵ�ص��ͭ�����ƽ�������6.4 g�����������ı�״����ˮú�������Ϊ ��
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�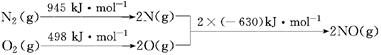
д���÷�Ӧ���Ȼ�ѧ����ʽ��________________��
��2��������(CH3OCH3)����ɫ���壬����Ϊһ��������Դ���ɺϳ���(���ΪH2��CO��������CO2)ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
(��)CO(g)��2H2(g)=CH3OH(g)��H1����90.1 kJ��mol��1
(��)CO2(g)��3H2(g)=CH3OH(g)��H2O(g)��H2����49.0 kJ��mol��1
ˮú���任��Ӧ��
(��)CO(g)��H2O(g)=CO2(g)��H2(g)��H3����41.1 kJ��mol��1
�����Ѻϳɷ�Ӧ��
(��)2CH3OH(g)=CH3OCH3(g)��H2O(g)��H4����24.5 kJ��mol��1
��H2��COֱ���Ʊ�������(��һ����Ϊˮ����)���Ȼ�ѧ����ʽΪ_______________��
���ݻ�ѧ��Ӧԭ������������ѹǿ��ֱ���Ʊ������ѷ�Ӧ��Ӱ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ�Ŀ����������������Ŀɳ�����չϢϢ��ء�
��.��֪��Fe2O3��s����3C��s��=2Fe��s����3CO��g��
��H1��a kJ��mol��1
CO��g����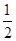 O2��g��=CO2��g���� ��H2��b kJ��mol��1
O2��g��=CO2��g���� ��H2��b kJ��mol��1
4Fe��s����3O2��g��=2Fe2O3��s���� ��H3��c kJ��mol��1
��C��ȼ���Ȧ�H��________kJ��mol��1��
��.��1������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���________������ţ���
A��C��s����CO2��g��=2CO��g��
B��NaOH��aq����HCl��aq��=NaCl��aq����H2O��l��
C��2H2O��l��=2H2��g����O2��g��
D��2CO��g����O2��g��=2CO2��g��
�������ڵ�K2CO3��CO2Ϊ��Ӧ�Ļ�����������ѡ��Ӧ��Ƴ�һ��ԭ��أ���д����ԭ��صĸ�����Ӧ��_____________________________________��
��2��ijʵ��С��ģ�ҵ�ϳɰ���ӦN2��g����3H2��g�� 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��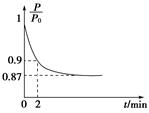
��ش��������⣺
�ٷ�Ӧ��ƽ��ı�־��__________________________������ĸ���ţ���ͬ����
A��ѹǿ���ֲ���
B�������ܶȱ��ֲ���
C��NH3������������N2���������ʵ�2��
��0��2 min�ڣ���c��N2���仯��ʾ��ƽ����Ӧ����Ϊ________________��
�������N2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________________________��
A������ϵ�а������1��1�ٳ���N2��H2
B�������NH3
C�������¶�
D�����뺤��ʹѹǿ����
E������һ������N2
��3��25��ʱ��BaCO3��BaSO4���ܶȻ������ֱ���8��10��9��1��10��10��ij����BaCO3����������Һ�У�c��CO32-����0.2 mol��L��1���������������Na2SO4��Һ����Ҫ����BaSO4����������Na2SO4��Һ�����ʵ���Ũ����С��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013������������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
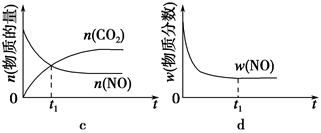
��1������β����������Ҫԭ��Ϊ2NO��g����2CO��g�� 2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
�ݴ��жϣ�
�ٸ÷�Ӧ�Ħ�H________0���>����<������
����T2�¶��£�0��2 s�ڵ�ƽ����Ӧ����v��N2����________��
�۵��������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2������ͼ�л���c��CO2����T1��S2�����´ﵽƽ������еı仯���ߡ�
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����________������ţ���
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
���磺CH4��g����2NO2��g��=N2��g����CO2��g����2H2O��g������H1����867 kJ��mol��1
2NO2��g�� N2O4��g������H2����56.9 kJ��mol��1
N2O4��g������H2����56.9 kJ��mol��1
д��CH4��g������ԭN2O4��g������N2��g����CO2��g����H2O��g�����Ȼ�ѧ����ʽ��__________________________________________________________________
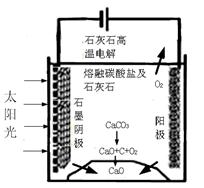
�ڽ�ȼú�����Ķ�����̼�������ã��ɴﵽ��̼�ŷŵ�Ŀ�ġ���ͼ��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪ_______________________________________��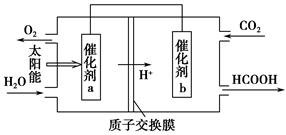
�۳����£�0.1 mol��L��1��HCOONa��ҺpHΪ10����HCOOH�ĵ��볣��Ka��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)(�㶫)����ʯ[��Ҫ�ɷ�Ca5(PO4)3F]�ڸ������Ʊ�����(P4)���Ȼ�ѧ����ʽΪ��4Ca5(PO4)3F(s)��21SiO2(s)��30C(s)===3P4(g)��20CaSiO3(s)��30CO(g)��SiF4(g)����H
��������Ӧ�У����������������________��
����֪��ͬ�����£�
4Ca5(PO4)3F(s)��3SiO2(s)===6Ca3(PO4)2(s)��2CaSiO3(s)��SiF4(g)����H1
2Ca3(PO4)2(s)��10C(s)===P4(g)��6CaO(s)��10CO(g)����H2
SiO2(s)��CaO(s)===CaSiO3(s)����H3
�æ�H1����H2�ͦ�H3��ʾ��H����H��____________��
(2)(����)��H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
��Cu(s)��2H��(aq)===Cu2��(aq)��H2(g) ��H1����64.39 kJ��mol��1
��2H2O2(l)===2H2O(l)��O2(g) ��H2����196.46 kJ��mol��1
��H2(g)��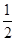 O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
��H2SO4��Һ�У�Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ʵ��Ļ�ѧ������ա�
��1��Na2CO3ˮ������ӷ���ʽ�� ��
��2��H2S���뷽��ʽ�� ��
��3��AlCl3ˮ������ӷ���ʽ�� ��
��4����25�桢101 kPa�£�l g������ȫȼ������CO2��Һ̬ˮʱ����55��6 kJ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ�� ��
��5����������ȼ�ϵ�ص������缫����ʽ
������ ��
������ ��
��6��д��NaHCO3��Һ�е�����Ũ�ȹ�ϵ
c(H+)+c(Na+)= ��
c(Na+)= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g)
FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g) CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g)
CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣���Դ�����ö�����̼�����ɼ�������������ŷţ��������»��ȼ�ϻ���Ҫ��ҵ��Ʒ��
��1����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO(NH2)2]����֪��
��2NH3��g���� CO2��g���� NH2CO2NH4��s�� ��H �� -159.47 kJ��mol-1
��NH2CO2NH4��s���� CO(NH2)2��s���� H2O��g�� ��H �� +116.49 kJ��mol-1
��H2O��l���� H2O��g�� ��H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ ��
��2����һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�
CO2(g)+4H2(g) CH4(g)+2H2O(g) ��H��0
CH4(g)+2H2O(g) ��H��0
����һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L��1��H2��0.8mol��L��1��CH4��0.8mol��L��1��H2O��1.6mol��L��1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ �� ��CO2��ƽ��ת����Ϊ ��
������������ͬ�ĺ��ݾ��ȣ������û�������������ܱ�����I��II����I�г���1 molCO2,��4 molH2����II�г���1 mol CH4��2 mol H2 O(g) ��300���¿�ʼ��Ӧ���ﵽƽ��ʱ������˵����ȷ���� ������ĸ����
| A������I��II������Ӧ������ͬ |
| B������I��II��CH4�����ʵ���������ͬ |
| C������I��CO2�����ʵ���������II�еĶ� |
| D������I��CO2��ת����������II��CH4��ת����֮��С��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�ֵ����Ϻ����ḻ��Ԫ�أ������仯������о���������������������Ҫ���塣
��1����ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��
��2����֪��N2(g)+ O2(g)��2 NO(g) ��H����180 kJ ? mol-1
2NO(g)+2 CO(g)��N2(g) + 2 CO2(g) ��H����746 kJ ? mol-1
��ӦCO(g) + O2(g)��CO2(g)�� ��H�� kJ ? mol-1��
O2(g)��CO2(g)�� ��H�� kJ ? mol-1��
��3����һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2 mol��N2��0.6 mol��H2����һ�������·������·�Ӧ�� N2(g)��3H2(g) 2NH3(g) ��H ��0�� ����5����ʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2 mol��ƽ��ʱH2��ת����Ϊ ��
2NH3(g) ��H ��0�� ����5����ʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2 mol��ƽ��ʱH2��ת����Ϊ ��
��4���ڹ̶�������ܱ������У�1.0��103 kPaʱ��������Ӧ N2(g)+3H2(g) 2NH3(g) ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g) ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 51 | K1 | K2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com