
��15�֣���Դ�����ö�����̼�����ɼ�������������ŷţ��������»��ȼ�ϻ���Ҫ��ҵ��Ʒ��
��1����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO(NH2)2]����֪��
��2NH3��g���� CO2��g���� NH2CO2NH4��s�� ��H �� -159.47 kJ��mol-1
��NH2CO2NH4��s���� CO(NH2)2��s���� H2O��g�� ��H �� +116.49 kJ��mol-1
��H2O��l���� H2O��g�� ��H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ ��
��2����һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�
CO2(g)+4H2(g) CH4(g)+2H2O(g) ��H��0
CH4(g)+2H2O(g) ��H��0
����һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L��1��H2��0.8mol��L��1��CH4��0.8mol��L��1��H2O��1.6mol��L��1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ �� ��CO2��ƽ��ת����Ϊ ��
������������ͬ�ĺ��ݾ��ȣ������û�������������ܱ�����I��II����I�г���1 molCO2,��4 molH2����II�г���1 mol CH4��2 mol H2 O(g) ��300���¿�ʼ��Ӧ���ﵽƽ��ʱ������˵����ȷ���� ������ĸ����
| A������I��II������Ӧ������ͬ |
| B������I��II��CH4�����ʵ���������ͬ |
| C������I��CO2�����ʵ���������II�еĶ� |
| D������I��CO2��ת����������II��CH4��ת����֮��С��1 |
��1��2NH3��g���� CO2��g����CO(NH2)2��s����H2O��l����H ����130.98 kJ��mol��1 ��3�֣�
��2����2mol��1�֣���8mol��1�֣���80% ��2�֣� ��CD��2�֣�
��3����̫���ܺ͵���ת��Ϊ��ѧ�ܣ�2�֣�
��2CO32����4e��=2CO2��+O2����2�֣���3CO2+4e��=C+2CO32����2�֣�
���������������1���ɢ٣��ڣ��ۿɵã�2NH3(g)�� CO2(g)��CO(NH2)2(s)��H2O(l) ��H ����130.98 kJ��mol��1 ��3�֣�
��2���ټ�����ʼ�Ķ�����̼�����ʵ���Ϊxmol�����������ʵ���Ϊymol��
CO2(g) + 4H2(g)  CH4(g) + 2H2O(g) ��H��0
CH4(g) + 2H2O(g) ��H��0
��ʼ���ʵ����� xmol ymol 0 0
ת�����ʵ����� 1.6mol 6.4mol 1.6mol 3.2mol
ƽ�����ʵ����� 0.4mol 1.6mol 1.6mol 3.2mol
��ô��x��0.4mol��1.6mol��2.0mol y��6.4mol��1.6mol��8.0mol
CO2��ƽ��ת����Ϊ��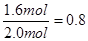
��A������I��II�е���ʼ��Ӧ�ﲻһ�����������Ӧ���ʲ�һ����ȣ�����B���������һ��������������ô�����������а����е�Ͷ���ǵ�Чƽ�⣬Ҳ����˵���������м���ĺ���Ӧ����ȣ�������һ�����ȵ�����������Ƕ�����̼���������ɼ����ˮ������Ӧ�Ƿ��ȷ�Ӧ���ų�������ʹ�����¶����ߣ������¶Ȼ�ʹ��ƽ�������ƶ���ʹ�ü���ĺ�����ǰ���ĵ�Чƽ��ʱ��Ҫ�ͣ���Ͷ������ˮ�����ɵ��Ƕ�����̼��������Ҫ���ȣ�ʹ����ϵ���¶Ƚ��ͣ����¶ȵĽ���ʹ��ƽ�������ƶ���ʹ�ü���ĺ�����ǰ���ĵ�Чƽ��ʱ��Ҫ�ߣ��������I��II��CH4�����ʵ�����������ͬ������C������I������ǰ����Чƽ��Ļ����������ƶ�������II������ǰ��ʶ����Чƽ��Ļ����������ƶ�����������I��CO2�����ʵ���������II�еĶ࣬��ȷ��D��������յ�Чƽ�������ǵĻ�������I��CO2��ת����������II��CH4��ת����֮�͵���1���������ھ��������У����ڵ�Чƽ��Ļ������ֱַ�������ƶ�����ˣ�����I��CO2��ת����������II��CH4��ת����֮��С��1����ȷ��
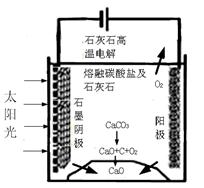
��3���ٴ�ͼ�п�֪װ���ǽ�̫���ܺ͵���ת��Ϊ��ѧ�ܣ�2�֣�
��������ʧȥ���ӵķ�Ӧ��2CO32����4e��=2CO2��+O2���������ǵõ����ӵķ�Ӧ���õ��ӵ�����ֻ���Ƕ�����̼��3CO2+4e��=C+2CO32����
���㣺���鷴Ӧ�ȡ���ѧƽ���Լ��绯ѧ���й�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡����ṩH2ȼ�շ�Ӧ���й����ʵĻ�ѧ�����ܣ� H��H������ΪQ1 kJ��mol��1��H��O������ΪQ3 kJ��mol��1��O2��������ԭ�Ӽ�ļ���ΪQ2 kJ��mol��1��
(1)������������ݣ���ͼ�б�ע�����ִ�����������仯����ֵ����ݼ�ͷ��ָ����д�����仯�ǡ��ų����������ǡ����ա�������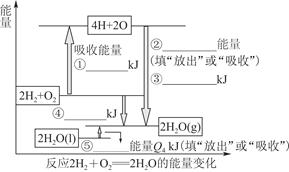
(2)��д��1 molH2ȼ������Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ�����ǵ�ǰ����������ٵ�һ���ش���⣬H2��CO��CH3OH������Ҫ����Դ���ʣ����ǵ�ȼ��������Ϊ285.8 kJ/mol��282.5 kJ/mol��726.7 kJ/mol����ش�
(1)��֪CO��H2��һ�������¿��Ժϳɼ״���CO+2H2=CH3OH����H2��CO��Ӧ����CH3OH���Ȼ�ѧ����ʽΪ�� ��
(2)��ͼΪij��ȼ�ϵ�صĹ���ԭ��ʾ��ͼ��a��b��Ϊ���Ե缫��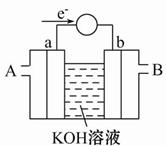
��ʹ��ʱ�������� ��ͨ��(�A����B��)��
�ڼ���ʹ�õġ�ȼ�ϡ��Ǽ״���a���ĵ缫��ӦʽΪ�� ________________
�ۼ���ʹ�õġ�ȼ�ϡ���ˮú��(�ɷ�ΪCO��H2)�����ֵ�ص��ͭ�����ƽ�������6.4 g�����������ı�״����ˮú�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���Ʊ� BaC12 �Ĺ�������ͼ��ͼ��ʾ��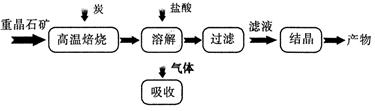
ij�о�С����ʵ�������ؾ�ʯ����Ҫ�ɷ�BaSO4���Թ�ҵ���̽���ģ��ʵ�顣����ã�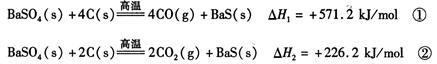
��1����ӦC(s) + CO2(g) 2CO(g)�ġ�H = kJ/mol
2CO(g)�ġ�H = kJ/mol
��2�����˹�������Ҫʹ�ò��������������������� ��
��3�������ܽⱺ�յĹ�������������ù��� NaOH ��Һ���գ��õ�������Һ�� Na2S ˮ������ӷ���ʽΪ ��
��4����BaCl2��Һ�м���AgNO3��KBr,�����ֳ�������ʱ��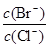 = ��[��֪��
= ��[��֪��
Ksp(AgBr) = 5.4��10��13 , Ksp(AgCl) = 2.0��10��10]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о���ѧ��Ӧ�е������仯����Ҫ���塣�����ѧ��֪ʶ�ش��������⣺
��1����֪һ����̼��ˮ������Ӧ���̵������仯����ͼ��ʾ��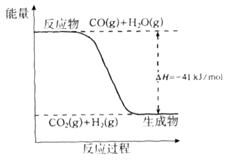
�ٷ�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
����֪��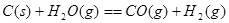
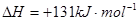
��
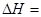
��2����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γɣ����1 mol��ѧ��ʱ�ͷţ������գ�����������֪��N��N���ļ�����948.9kJ��mol��1��H��H���ļ�����436.0 kJ��mol��1�� N��H���ļ�����391.55 kJ��mol��1����1/2N2(g) + 3/2H2(g) ="=" NH3(g) ��H = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����(COCl2)�����ϡ��Ƹ��ҩ�ȹ�ҵ����������;����ҵ�ϲ��ø�����CO��Cl2�ڻ���̿���ºϳɡ�
��1��������ҵ����Դ���ȼҵ���ȼҵ�Ļ�ѧ����ʽΪ
��2����ҵ��������Ȼ��(��Ҫ�ɷ�ΪCH4)��CO2���и��������Ʊ�CO��H2����֪CH4��H2��CO��ȼ����(��H)�ֱ�Ϊ��890.3 kJ/mol����285.8kJ/mol�ͣ�283.0 kJ/mol����÷�Ӧ���Ȼ�ѧ����ʽΪ��_____ _____��
��3��COCl2�ķֽⷴӦΪCOCl2(g)  Cl2(g) + CO(g) ��H =" +108" kJ/mol����Ӧ��ϵ�ﵽƽ������ʵ�Ũ���ڲ�ͬ�����µı仯״������ͼ��ʾ(��10 min��14 min��COCl2Ũ�ȱ仯������ʾ��)��
Cl2(g) + CO(g) ��H =" +108" kJ/mol����Ӧ��ϵ�ﵽƽ������ʵ�Ũ���ڲ�ͬ�����µı仯״������ͼ��ʾ(��10 min��14 min��COCl2Ũ�ȱ仯������ʾ��)��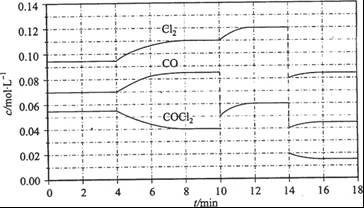
�ټ��㷴Ӧ�ڵ�8 minʱ��ƽ�ⳣ��K = __________(����С�������λ����)
�ڱȽϵ�2 min��Ӧ�¶�T��2�����8 min��Ӧ�¶�(T8)�ĸߵͣ�T��2��____T��8��(�<���� ��>����=��)��
����12 minʱ��Ӧ���¶�T��8�������´ﵽƽ�⣬���ʱc(COCl2) = ______mol/L��
�ܱȽϲ���CO��2��3 min��5��6 min��12��13 minʱƽ����Ӧ����[ƽ����Ӧ���ʷֱ���V(2��3)�� V(5��6)�� V(l2��13)��ʾ]�Ĵ�С____________��
�ݷ�Ӧ��COCl2��5��6 min��15��16 minʱƽ����Ӧ���ʵĴ�СΪ��V(5��6) > V(15��16)��ԭ����__ _____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��д�����з���
��1��̼������Һ�ʼ��Ե�ԭ�������ӷ��̱�ʾ ��
��2����п������������ʩ�����и��������ĵ缫��ӦΪ�� ��
��3����20.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7 kJ�����������ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ ��
��4��������þ�ܽ���Ũ���Ȼ����Һ�������ӷ��̱�ʾ ��
��5�� Al��OH��3�ĵ��뷴Ӧ����ʽ: ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����̲��Ŵ����ġ���ȼ�������ü�����ˮú����CO��H2�����ٺϳɼ״����������湩Ӧ���ŵ�ȼ�͡�
��֪���� CH4(g)��H2O (g)��CO (g)��3H2 (g) ��H1��+206.2kJ��mol-1
�� CH4(g)��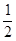 O2(g)��CO(g)��2H2(g) ��H2=��35.4 kJ��mol-1
O2(g)��CO(g)��2H2(g) ��H2=��35.4 kJ��mol-1
�� CH4 (g)��2H2O (g)��CO2 (g)��4H2 (g) ��H3��+165.0 kJ��mol-1
��1��CH4(g)��CO2 (g)��Ӧ����CO(g)��H2(g)���Ȼ�ѧ����ʽΪ ��
��2����ԭ�ϡ���Դ���õĽǶȣ�������Ӧ����Ϊ�ϳɼ״������˷�����ԭ���� ��
��3��ˮú���е�H2����������NH3���ڽ���ϳ���ǰ����[Cu(NH3)2]Ac��Һ���������е�CO����ֹ�ϳ����еĴ����ж����䷴Ӧ�ǣ� [Cu(NH3)2]Ac + CO + NH3  [Cu(NH3)3]Ac��CO ��H��0
[Cu(NH3)3]Ac��CO ��H��0
[Cu(NH3)2]Ac��Һ����CO��������������Ӧ�� ��
��4����CH4��Ƴ�ȼ�ϵ�أ��������ʸ��ߣ�װ��ʾ������ͼ��A��BΪ�����ʯī����������ͨ����飬�ڱ�״���£����ļ������VL��0��V��44.8 Lʱ������ܷ�Ӧ����ʽΪ ��
�� 44.8 L��V��89.6 Lʱ�������缫��ӦΪ ��
�� V="67.2" Lʱ����Һ������Ũ�ȴ�С��ϵΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��I����ͼ�ǹ�ҵ��������淋����̡�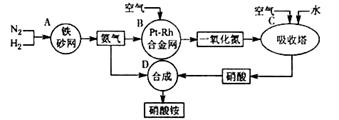
��1��������C��ͨ�������Ŀ���� ��A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ���� ������ĸ����
��2����֪��4NH3(g) + 3O2(g) = 2N2(g) +6H2O(g) ��H =��1266.8kJ/mol
N2(g) + O2(g) = 2NO(g) ��H =" +180.5" kJ/mol
д�������´��������Ȼ�ѧ����ʽ��
��II��ij����С��ͬѧ��ͭƬ����ϡ���ᣬ���ֿ�ʼʱ��Ӧ�dz�����һ��ʱ���Ӧ�������Լӿ졣��С��ͨ��ʵ��̽����ԭ��
��3���÷�Ӧ�����ӷ���ʽΪ___________________________________________________��
��4������������衣��ʵ���з�Ӧ�������Լӿ��ԭ�������_____________________��
A����Ӧ���ȵ����¶����� B��ѹǿ����
C���������д����� D����Ӧ��Ӵ��������
��5������̽�����ⶨ��Ӧ��������Һ��ͬʱ����¶ȣ�������±���
| ʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 35 | 50 | 60 | 70 | 80 |
| �¶�/�� | 25 | 26 | 26 | 26 | 26 | 26 | 26.5 | 27 | 27 | 27 | 27 |
| ʵ�� ��� | ͭƬ ����/g | 0.1mol��L-1�� �������/mL | ����ͭ ����/g | �������� ����/g | ʵ��Ŀ�� |
| �� | 5 | 20 | _______ | _______ | ʵ��ٺ͢�̽��_________��Ӱ�죻ʵ��ٺ͢�̽�����������Ӱ�졣 |
| �� | 5 | 20 | 0.5 | 0 | |
| �� | 5 | 20 | 0 | 0.5 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com