
��ʵ���ҿ���ͭ��Ũ������Ȼ�������������Ʒ�Ӧ��ȡ�������������������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ���ͼ�п�ѡ�õķ���װ���� ����д��ĸ����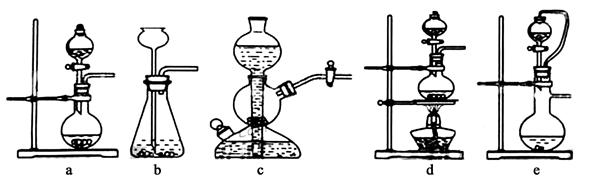
��Aͼ��ʾijѧ����SO2��Ư�۾�[80%Ca(ClO)2��]�ķ�Ӧ����ʵ��̽���Ĺ��̣��۲쵽�������У�
��.Һ���Ϸ����ְ�����
���Ժ��ֻ��ǣ���Һ��Ϊ����ɫ��
���Ժ���������ɫ����������ɫ��ȥ
��1����ˮ�г���ͨ��SO2��δ�۲쵽�������Ʋ�������еİ�����HClСҺ���γɣ���������ʵ�飺
a����ʪ��ĵ⻯�ص�����ֽ����������ޱ仯��
b�����ữ��AgNO3��Һ���������������ɫ������
��ʵ��a��b�����жϰ����к���HCl�������� ��
��2�����д�����ɫ�����ijɷ��� ��
��3�����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾�����Ч�ɷֺ�C1-������Ӧ������Cl2��ͨ����һ��ʵ��ȷ�������ֿ����ԣ���ʵ�鷽���� ��
��4�������ӷ���ʽ����������л���ɫ��ȥ��ԭ�� ��
��5��Bͼ��ʾʯ��-ʯ�෨����SO2�Ĺ������̣�д����Ӧ�Ļ�ѧ����ʽ��
��
��ae (2��)
��.�� �����к���SO2Ҳ�����ữ��AgNO3��Ӧ���ɰ�ɫ����(2��)
��CaSO4 (2��)
�ǿ�ȡ����ԭ��Һ����һ������ϡ����۲쵽��Һ�����ɫ(2��)
�� Cl2+SO2+2H2O=4H++2Cl-+SO42- (2��)
�� SO2+Ca(OH)2=CaSO3��+H2O ��2CaSO3+O2+4H2O=2{CaSO4��2H2O��}(2��)
�����������������������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ����ڷ�Ӧ����Ҫ���ȣ��ų�װ��d����������������ϸС����������ѡ��װ��bc���ʿ�ѡ�õķ���װ����ae�����������к���SO2��Ҳ�����ữ��AgNO3��Ӧ���ɰ�ɫ������������SO2��Ҳ������Ϊ���ᣬ����ʹ�����Ʒ������ֽⷴӦ��������ư�ɫ��������ȡ����ԭ��Һ����һ������ϡ���ᣬCl-��ClO-��H+�������з�Ӧ�õ�����ɫ��������ʹ��Һ�����ɫ����������Cl2�ͻ�ԭ�Ե���SO2��ˮ��Һ�з���������ԭ��Ӧ������������ᣬ�Ӷ�ʧȥ�����Ļ���ɫ��Cl2+SO2+2H2O=4H++2Cl-+SO42-��ʯ��-ʯ�෨����SO2�ĵĻ�ѧ����ʽSO2+Ca(OH)2=CaSO3��+H2O ��2CaSO3+O2+4H2O=2{CaSO4��2H2O}��
���㣺����ʵ������ȡ����Ӧ�ÿ��ǵ����ء������Ļ�ѧ���ʺ�Ӧ�ã��������SO2Σ�������Ϊ����


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣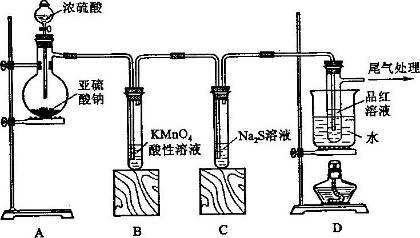
��ش��������⣺
��1��װ��A��ʢ���������Ƶ�����������_________�����з�����Ӧ�Ļ�ѧ����ʽ_______________________��
��2��ʵ������У�װ��B��C�з���������ֱ���____________��___________��װ��B�з�����Ӧ�����ӷ���ʽΪ_________________________________��
��3��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������________________________��
��4��β���ɲ���___________________��Һ���ա�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧ��ȤС�������ͼʾʵ��װ�ã�ͼ��ʡ���˼г����������ⶨij��̼�Ͻ�������������������̽������Ũ����ķ�Ӧ��
��1��m����̼�Ͻ��м������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���Ǣٳ�����̼��Ũ�����Ӧ���� ��
��2��д������ʱA��̼��Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽ ��
��3��B�е������ǣ� ��
C�������ǣ� ��
��4����A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����أ�E����bg������̼�Ͻ���������������Ϊ (д��m��b�ı���ʽ)��
�ɷ�Ӧһ��ʱ����õι���ȡA�е���Һ���뵽����ˮ����Ϊ�����������������������ӵijɷ����������ֿ���:
A��ֻ����Fe3+��B��ֻ����Fe2+��C�� ��
��֤C��ʵ�鷽����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧУ��ѧѧϰС��Ϊ̽���������������ʣ�����ͼ��ʾװ�ý���ʵ�飬��ش���������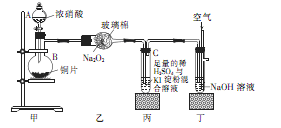
��1��װ�ü���ʢ��Ũ���������A�������� ������B�з�����Ӧ�Ļ�ѧ����ʽΪ
��2�������������еĿհ�
��3��ȡ��װ�ñ��е��Թ�C�������еμ�������Na2SO3��Һ����Һ��ɫ��ȥ���ù����з�����Ӧ�����ӷ���ʽΪ ����Ӧ�����Һ����Ҫ����SO32����SO42����I���������ӣ�����д����SO32����SO42����I����ʵ�鱨�档
��ѡ�Լ���2mol��L��1HCl��1mol��L��1H2SO4��1mol��L��1BaCl2��1mol��L��1Ba(NO3)2��CCl4�����Ʊ�����ˮ�����Ʊ�����ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�� (1)ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ (NH4)2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a 
(2)��װ��C������Һ����뿪�IJ���������__ _______��
װ��D�������� ��
��4�֣�Ϊ����Ȼ�淋ľ��ü�ֵ���ҹ���ѧ�����������������þ�ȷֽ��Ȼ���ư������õ���ʽ�Ȼ�þ(MgOHCl)�Ĺ��ա�ijͬѧ���ݸ�ԭ����Ƶ�ʵ��װ����ͼ��
��ش��������⣺
(1) װ��A�з�����Ӧ���ɼ�ʽ�Ȼ�þ�Ļ�ѧ����ʽΪ_________ ____
װ��B�м�ʯ�ҵ�������_____ __
(2) ��Ӧ�����г���ͨ��N2�����������㣺һ��ʹ��Ӧ�����İ�����ȫ��������ϡ���������գ�����_______________ ______
(3) װ��C���Թ��з�Ӧ�����ӷ���ʽΪ ______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС���һ�������ǡ�NO2�ܷ�֧��ľ����ȼ�գ�������ʵ������û���ֳɵ�NO2���壬��С���ͬѧ�������������������N2O4�Ĵ��ڣ�ͼ������̨�ȼг�����������ȥ����
��ʵ��װ������ͼ1��ʾ
ͼ1 ͼ2
��1��Ũ�������ȷֽ�Ļ�ѧ����ʽ�� ��
��2��ʵ�鿪ʼ��ľ���ϵĻ�����Ϩ���е�ͬѧ�ó���NO2����֧��ľ����ȼ�ա��Ľ��ۡ�����Ϊ��һ�����Ƿ���ȷ�� �����ȷ������ȷ���������� ��
��ʵ��װ������ͼ2��ʾ
��1������ͭ������ȷֽ�IJ���������ͭ�������������������÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��������ƿ�г�������ɫ����ʱ��ľ����ȼ�ˣ��е�ͬѧ�ó���NO2��֧��ľ����ȼ�ա��Ľ��ۡ�����Ϊ��һ�����Ƿ���ȷ�� �����ȷ������ȷ������������ ��
��Ϊ�˸�ֱ��˵����NO2�ܷ�֧��ľ����ȼ�ա���һ���⣬�����������һ����ʵ�鷽��������ʵ��ԭ������Ҫ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ڴ�ŵ��������ƿ��ܻᱻ�����е�����������ij��ѧ��ȤС��ͨ��ʵ�����ⶨ���������Լ��������ij̶ȣ��������ͼʵ�飬��ش���������⣺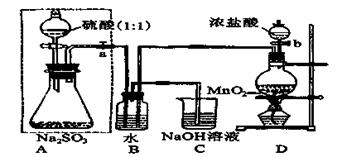
(1)Dװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
Bװ���з�Ӧ�����ӷ���Ϊ ��
(2)����ag Na2SO3��Ʒ������ƿ�У���Bװ�÷�Ӧ�����Һ�м���������BaCl2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ�����ð�ɫ����bg��ԭ��Ʒ��Na2SO3��������Ϊ��Ϊ�� ��
(3)Ϊ��֤ʵ��ⶨ��ȷ�ԣ�A�е�����ʲôʱ��μ� ��
Cװ����NaOH��Һ�������� _________________��
(4)���������Լ���������ˮ����ϡ���ᡢ��ϡ���ᡢ��BaCl2��Һ����Ba(NO3)2��Һ�������ѡ������Լ������һ�ֲ�ͬ��ʵ�鷽�����ⶨ��������ˮ�������Ʊ������ij̶ȣ���ʹ���Լ���˳��Ϊ�� ��(���Լ����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС������ͼһװ����ȡ������̽���������й����ʡ�
��1��װ��A����ƿ���Լ���ѡ��________������ţ�
a����ʯ�� b��Ũ���� c����ʯ�� d������������ e���ռ�
��2����̽���������ܽ��ԣ�����K2�ĵ���ĩ������ͼ��װ���е�_____װ�ã�����ţ�����װ��D�м��������ر�K1��K2����K3��������Ȫ��ʵ�������______________
___________________________________________________________________________��
��3����̽�������Ļ�ԭ�ԣ����K1��K2��K3������������ȡ����������������װ�á�
���ö���������Ũ������ȡ�����������������ͨ��ʢ��____________�Լ���ϴ��ƿ��
��D�а�����������Ӧ�������̣�ͬʱ����һ����ɫ��ζ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
�۴�K3�������ݳ��������к�������Cl2����Cװ����Ӧʢ��_________��Һ���ѧʽ������Ӧ�����ӷ���ʽΪ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
X��Y��Z�����ֳ����ĵ��ʣ��ס��������ֳ����Ļ�����±���������֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת������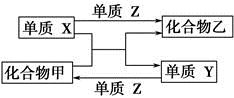
| ѡ�� | X | Y | Z | �� | �� |
| A | H2 | Si | Cl2 | SiCl4 | HCl |
| B | Mg | C | O2 | CO2 | MgO |
| C | Zn | Fe | Cl2 | FeCl2 | ZnCl2 |
| D | Cl2 | N2 | H2 | NH3 | HCl |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com