
�� (1)ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ (NH4)2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a 
(2)��װ��C������Һ����뿪�IJ���������__ _______��
װ��D�������� ��
��4�֣�Ϊ����Ȼ�淋ľ��ü�ֵ���ҹ���ѧ�����������������þ�ȷֽ��Ȼ���ư������õ���ʽ�Ȼ�þ(MgOHCl)�Ĺ��ա�ijͬѧ���ݸ�ԭ����Ƶ�ʵ��װ����ͼ��
��ش��������⣺
(1) װ��A�з�����Ӧ���ɼ�ʽ�Ȼ�þ�Ļ�ѧ����ʽΪ_________ ____
װ��B�м�ʯ�ҵ�������_____ __
(2) ��Ӧ�����г���ͨ��N2�����������㣺һ��ʹ��Ӧ�����İ�����ȫ��������ϡ���������գ�����_______________ ______
(3) װ��C���Թ��з�Ӧ�����ӷ���ʽΪ ______________
��1��d e f ��2�� ��Һ�����ն����NH3��ֹ��Ⱦ��������ֹ����
�� ��1��Mg(OH)2+NH4Cl MgOHCl+NH3��+H2O ���ﰱ��
MgOHCl+NH3��+H2O ���ﰱ��
��2����ֹװ��C�е�AlCl3��Һ������װ��B ��3�� Al3++3NH3��H2O=Al(OH)3��+3NH4+
���������������1����Aװ����ȡ�İ���ͨ�뵽�����пƵ���������ʡ�Ϊ�˷�ֹ����İ�����Ⱦ������Ҫ�������õ��۵�©��ͨ�뵽ˮ�С������ȿ���ʹ����������գ�Ҳ��ֹ�˵�������ķ���������װ�ýӿ�������d e f����������Ȼ�̼�ǻ������ܵ�����Һ�壬���÷�Һ�����롣(1) װ��A�з�����Ӧ���ɼ�ʽ�Ȼ�þ�Ļ�ѧ����ʽΪMg(OH)2+NH4Cl MgOHCl+NH3��+H2O װ��B�м�ʯ�ҵ����������հ����е�ˮ������ ���ﰱ����(2) ��Ӧ�����г���ͨ��N2�����������㣺һ��ʹ��Ӧ�����İ�����ȫ��������ϡ���������գ����Ƿ�ֹװ��C�е�AlCl3��Һ���ڰ����Ĵ����ܽ���ɵ�װ���е�ѹǿ��С����ĵ�������ʹAlCl3��Һ������װ��B��(3) װ��C���Թ��з�Ӧ�����ӷ���ʽΪAl3++3NH3��H2O=Al(OH)3��+3NH4+ ��
MgOHCl+NH3��+H2O װ��B�м�ʯ�ҵ����������հ����е�ˮ������ ���ﰱ����(2) ��Ӧ�����г���ͨ��N2�����������㣺һ��ʹ��Ӧ�����İ�����ȫ��������ϡ���������գ����Ƿ�ֹװ��C�е�AlCl3��Һ���ڰ����Ĵ����ܽ���ɵ�װ���е�ѹǿ��С����ĵ�������ʹAlCl3��Һ������װ��B��(3) װ��C���Թ��з�Ӧ�����ӷ���ʽΪAl3++3NH3��H2O=Al(OH)3��+3NH4+ ��
���㣺�������������ӡ������ķ��뼰��������ȡ�����ʡ������֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������һ����Ҫ�ķǽ������ϣ��Ʊ��������Ҫ�������£�
�ٸ�������̼��ԭ���������Ƶôֹ�
�ڴֹ������HCl���巴Ӧ�Ƶ�SiHCl3��Si��3HCl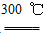 SiHCl3��H2
SiHCl3��H2
��SiHCl3�����H2��1 000��1 100 �淴Ӧ�Ƶô��衣
��֪SiHCl3����H2Oǿ�ҷ�Ӧ���ڿ���������ȼ��
��ش��������⣺
(1)�ڢٲ��Ʊ��ֹ�Ļ�ѧ��Ӧ����ʽΪ________________________________��
(2)�ֹ���HCl��Ӧ��ȫ�������õ���SiHCl3(�е㣭33 ��)�к�������SiCl4(�е�57.6 ��)��HCl(�е㣭84.7 ��)���ᴿSiHCl3���õķ���Ϊ________��
(3)��SiHCl3�����H2��Ӧ�Ʊ������װ������ͼ(��Դ���г�װ����ȥ)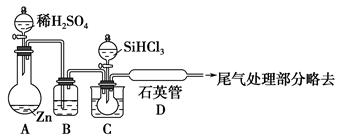
��װ��B�е��Լ���________��װ��C�е���ƿ��Ҫ���ȣ���Ŀ����___________________________________________________________��
�ڷ�Ӧһ��ʱ���װ��D�й۲쵽��������________��װ��D���ܲ�����ͨ�����ܵ�ԭ����__________________________________________________��
װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��Ϊ��֤�Ʊ�����ʵ��ijɹ��������Ĺؼ��Ǽ��ʵ��װ�õ������ԣ����ƺ÷�Ӧ�¶��Լ�________________________________________________��
��Ϊ������Ʒ�����Ƿ��������ʣ���������ϡ�����ܽ⣬ȡ�ϲ���Һ�����ټ�����Լ���(��д��ĸ����)________��
a����ˮ��b����ˮ��c��NaOH��Һ��d��KSCN��Һ e��Na2SO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧϰС��̽��Ũ��ϡ���������Ե����ǿ��������ͼװ�ý���ʵ�飨�г���������ȥ����
��ѡҩƷ��ϡ���ᡢŨ���ᡢŨ���ᡢNaOH��Һ������ˮ
�������ϣ�
A��Ũ�����ܽ�NO������NO2����ϡ���������NO��
B������������Һ����NO��Ӧ������NO2��Ӧ2NO2 + 2NaOH = NaNO3 + NaNO2 +H2O
| ʵ����� | ʵ������ |
| I������װ�õ������� | |
| II������ | |
| III����Һ©����������Ũ���Ỻ��������ƿ�У��رջ����� | ���в��������ĺ���ɫ���壬����ɫ�����ڢ��б�Ϊ��ɫ������ͨ���ۺ���ȻΪ��ɫ��ͨ���ܺ��Ϊ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ�У�A�������ķ���װ�ã�B��C�Ǿ��������װ�ã�D��װ��˿������Ӧ��E�ĵײ�����ɫ����ۼ���F�����ն��������װ�á�
��1������װ������һ��������ָ���� ��������ĸ��ʾ����
��2��ͨ��B��Ϊ�˳�ȥ ����B��Ӧ���� ��ͨ��C��Ϊ�˳�ȥ ��
��3��д��D��F�з�����Ӧ�Ļ�ѧ����ʽ �� ��
��4�����A�в�������3.36L����״����������㣺
��д��A�з�����Ӧ�Ļ�ѧ����ʽ�������A�еĵ���ת����� ��
������MnO2�����ʵ��� ���۱�������HCl�����ʵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС��������װ�ó�ȡ�ռ����������������о������ʡ�������������⡣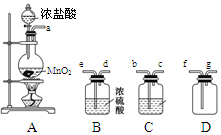
��1��װ��A�з�����Ӧ�����ӷ���ʽΪ_______________________________��
��2��������������������ӿڵ�����˳��Ϊa��___________________��g��
��3��װ��B��Ũ�����������____________________________________________________________��װ��C���Լ������___________________________________��
��4��ijͬѧ��Ϊ��������ȱ��β������װ�ã���������ķ����л�����װ�ò�ע���Լ���
| |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
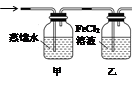
��ʵ���ҿ���ͭ��Ũ������Ȼ�������������Ʒ�Ӧ��ȡ�������������������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ���ͼ�п�ѡ�õķ���װ���� ����д��ĸ����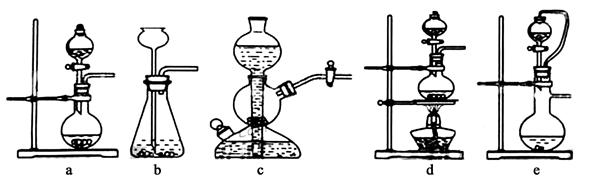
��Aͼ��ʾijѧ����SO2��Ư�۾�[80%Ca(ClO)2��]�ķ�Ӧ����ʵ��̽���Ĺ��̣��۲쵽�������У�
��.Һ���Ϸ����ְ�����
���Ժ��ֻ��ǣ���Һ��Ϊ����ɫ��
���Ժ���������ɫ����������ɫ��ȥ
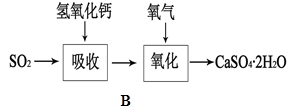
��1����ˮ�г���ͨ��SO2��δ�۲쵽�������Ʋ�������еİ�����HClСҺ���γɣ���������ʵ�飺
a����ʪ��ĵ⻯�ص�����ֽ����������ޱ仯��
b�����ữ��AgNO3��Һ���������������ɫ������
��ʵ��a��b�����жϰ����к���HCl�������� ��
��2�����д�����ɫ�����ijɷ��� ��
��3�����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾�����Ч�ɷֺ�C1-������Ӧ������Cl2��ͨ����һ��ʵ��ȷ�������ֿ����ԣ���ʵ�鷽���� ��
��4�������ӷ���ʽ����������л���ɫ��ȥ��ԭ�� ��
��5��Bͼ��ʾʯ��-ʯ�෨����SO2�Ĺ������̣�д����Ӧ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ClO2��Ϊ�����������������ж��������������й㷺��Ӧ��ǰ����ijͬѧ����ͼ��ʾ��װ���Ʊ�ClO2���壬��Ӧԭ��Ϊ���Ͳ�����Һ��KClO3��ĩ��60��ʱ��Ӧ�Ƶ�ClO2���¶ȹ�����Ͷ���Ӱ������Ч�ʣ�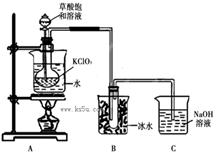
��֪��Ϣ����ClO2��һ�ֻ���ɫ�д̼�����ζ�����壬�۵�-59�棬�е�11.0�档��Ӧ���������ơ�
�ڲ���������ǿ�ڴ���Ķ�Ԫ���ᣬ��Ӧ�ĸ��Σ�CaC2O4�������ڴ��ᣬ������ǿ�ᣬ������һ�ֻ�ԭ�Խ�ǿ�����ʡ�
��1���Ʊ�ClO2�Ļ�ѧ����ʽ��2KClO3+H2C2O4= 2KHCO3+2ClO2��������˵����ȷ����
| A��KClO3�ڷ�Ӧ�еõ����� |
| B��ClO2���������� |
| C��H2C2O4�ڷ�Ӧ��ʧȥ���� |
| D��1mol KClO3�μӷ�Ӧ��2mol����ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������һ�ִ�����Ⱦ��о���NO2��SO2��CO�ȴ�����Ⱦ����Ĵ�������Ҫ���壬ij��ѧʵ�鰮��С����̽��SO2�����ʣ�������·�����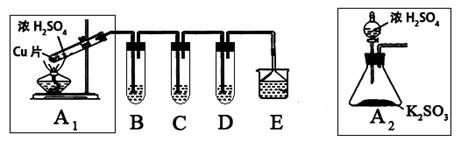
��1��B��C��D�ֱ����ڼ���SO2�Ļ�ԭ�ԡ������Ժ�Ư���ԡ�����B��C�ֱ�Ϊ��ˮ�������ˮ��Һ����D����ʢ�Լ�Ϊ_________��B�з�Ӧ�����ӷ���ʽΪ��_________________��
��2��Ϊ��ʵ����ɫʵ���Ŀ�꣬ijͬѧ���������������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��ǣ�________________________________����дһ�㼴�ɣ���
��3��E���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH����SO32����SO42����HSO3���������ӡ���֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2mol/L���ᡢ1mol/L BaCl2��Һ��1mol/L Ba(OH)2��Һ��Ʒ����Һ������ˮ��
�����ʵ��֤��������Һ���д���SO32����HSO3��������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡ1mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�����Һ�д���SO32���� SO42���� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� ___________________________________________________ | _________________________ ______________________________________________ |
| ����3��_______ _______________________ ___________________________________________________ | _________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
A��B��C�ֱ���Ԫ�ؼס��ҡ����ĵ��ʣ����Ƕ��dz����Ľ�����ǽ�����D��E��F�dz��������������������ͼ��ʾת����ϵ��������˵������ȷ����(����)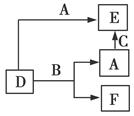
A��D��E��һ�������м�Ԫ��
B������B�϶���������
C��A��B��C��һ����һ������Ԫ�صĵ���
D����A�Ƿǽ�������Bһ��Ϊ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com