
����Ŀ��![]() ������ij��Һ����ˮ�����������Ũ�ȷ���
������ij��Һ����ˮ�����������Ũ�ȷ���![]() ����Һ����pHΪ ______ ����ʱˮ�ĵ����ܵ� ______ ��
����Һ����pHΪ ______ ����ʱˮ�ĵ����ܵ� ______ ��
![]() ��֪��
��֪��![]() һ���¶��£����ܱ������з�Ӧ
һ���¶��£����ܱ������з�Ӧ![]() �ﵽƽ�⣮������������ʱ�����д�ʩ�����
�ﵽƽ�⣮������������ʱ�����д�ʩ�����![]() ת���ʵ��� ______
ת���ʵ��� ______ ![]() ����ĸ
����ĸ![]() ��
��
A.��С![]() ��Ũ��
��Ũ��![]() �����¶�
�����¶�![]() ����
����![]() ��Ũ��
��Ũ��![]() �����¶�
�����¶�
![]() ��ij�¶��£�
��ij�¶��£�![]() �����ӻ�����Ϊ
�����ӻ�����Ϊ![]()
![]() ������¶��£�
������¶��£�
![]() ��Һ��
��Һ��![]() ______ ��
______ ��
![]()
![]() ��Һ��100mL
��Һ��100mL![]() ��KOH��Һ��Ϻ�
��KOH��Һ��Ϻ�![]() _____
_____
![]() ��֪һ��Һ��4�����ӣ�
��֪һ��Һ��4�����ӣ�![]() ��
��![]() ��
��![]() ��
��![]() �����з�������϶�������� ______
�����з�������϶�������� ______
A.![]()
![]()
C.![]()
![]()
![]() ��
��![]() �£���a
�£���a![]() �İ�ˮ��
�İ�ˮ��![]() ������������ϣ���Ӧʱ��Һ��
������������ϣ���Ӧʱ��Һ��![]() ����Һ�� ______
����Һ�� ______ ![]() ����������������������
����������������������![]() �ԣ��ú�a�Ĵ���ʽ��ʾ
�ԣ��ú�a�Ĵ���ʽ��ʾ![]() �ĵ��볣��
�ĵ��볣��![]() ______ ��
______ ��
���𰸡�![]() ��10 ���� BC 11 12 C ��
��10 ���� BC 11 12 C �� ![]()
��������
(1)������ij��Һ����ˮ�����������Ũ�ȷ���c(H+)c(OH-)=1��10-20����Һ�����㣺c(H+)=c(OH-)=1��10-10mol/L�������Һ������ˮ�ĵ��룬Ϊ���Ի������Һ��
(2)�÷�Ӧ�Ƿ�Ӧǰ�����������С�ġ����ȵĿ��淴Ӧ��Ҫʹ�÷�Ӧ������Ӧ�����ƶ����ɸı䷴Ӧ���Ũ�ȡ���ϵ��ѹǿ���¶ȵȣ����ݻ�ѧƽ���ƶ�ԭ������ѡ�
(3)����ij�¶��£�H2O�����ӻ�����Ϊ1��10-13mol2L-2��0.01molL-1NaOH��Һ������������Ũ��Ϊ0.01molL-1����������Ũ��Ϊ10-11mol/L���ݴ˼�����ҺpH��
��100mL 0.1molL-1H2SO4��Һ��100mL 0.4molL-1��KOH��Һ��Ϻ�ǡ��Ӧ������������Һ������������Ũ�ȹ�������Һ�Լ��ԣ�����ʣ������������Ũ�Ⱥ����ӻ�����������Һ��������Ũ�ȼ�����ҺpH��
(4)����Һ����Ϊ���ԡ����ԡ�������Һ������Һ��һ���������غ㣬���ݵ���غ�����жϣ�
(5)��25���£�ƽ��ʱ��Һ��c(NH4+)=c(Cl-)=0.005mol/L�����������غ��c(NH3��H2O)=(0.5a-0.005)mol/L�����ݵ���غ��c(H+)=c(OH-)=10-7mol/L����Һ�����ԣ�����NH3H2O�ĵ��볣��Kb���㡣
(1)������ij��Һ����ˮ�����������Ũ�ȷ���c(H+)c(OH-)=1��10-20����Һ�����㣺c(H+)=c(OH-)=1��10-10mol/L�������Һ������ˮ�ĵ��룬Ϊ���Ի������Һ��������Һ��pH����Ϊ4��10��
(2)�÷�Ӧ������Ӧ�����������С�ġ����ȷ�Ӧ��Ҫʹ��Ӧ������Ӧ�����ƶ����ɸı䷴Ӧ���Ũ�ȡ���ϵ��ѹǿ���¶ȵȡ�
A.��СNO2��Ũ�ȣ�ƽ�����淴Ӧ�����ƶ���NO2��ת���ʽ��ͣ�A����
B.�����¶ȣ�ƽ��������Ӧ�����ƶ���NO2��ת������ߣ�B��ȷ��
C.����NO2��Ũ�ȣ�ƽ��������Ӧ�����ƶ�������ϵѹǿ����Ҳ���ڷ�Ӧ������Ӧ�����ƶ���C��ȷ��
D.�����¶ȣ�ƽ�����淴Ӧ�����ƶ���NO2��ת���ʽ��ͣ�D����
�ʺ���ѡ����BC��
(3)����Һ��c(H+)=![]() mol/L=1��10-11 mol/L��pH=-lgc(H+)=-lg10-11=11��
mol/L=1��10-11 mol/L��pH=-lgc(H+)=-lg10-11=11��
�ڻ�Ϻ���������Һ�Լ��ԣ��ڻ�Ϻ����Һ��c(OH-)=![]() =0.1mol/L��c(H+)=
=0.1mol/L��c(H+)=![]() mol/L=10-12 mol/L��pH=-lgc(H+)=12��
mol/L=10-12 mol/L��pH=-lgc(H+)=12��
(4)A.����ҺΪ����ʱ�����������ϵ��c(Y-)>c(X+)>c(H+)>c(OH-)��A��ȷ��
B.����Һ�ʼ���ʱ��������c(X+)>c(Y-)>c(OH-)>c(H+)��B��ȷ��
C.�ù�ϵc(H+)>c(Y-)>c(X+)>c(OH-)���������غ㣬C����
D.����Һ�ʼ��ԣ���YOHԶԶ����ʱ��������c(OH-)>c(X+)>c(H+)>c(Y-)��D��ȷ��
�ʺ���ѡ����C��
(5)��25���£�ƽ��ʱ��Һ��c(NH4+)=c(Cl-)=0.005mol/L�����������غ��c(NH3H2O)=(0.5a-0.005)mol/L�����ݵ���غ��c(H+)=c(OH-)=10-7mol/L����Һ�����ԣ�NH3H2O�ĵ��볣��Kb=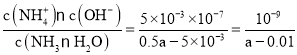 ��
��


 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д� ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ڹ�ũҵ�����ж�����ҪӦ�á�
(1)������(N2H4)����������ĵ��⻯�
��֪��4NH3(g)��3O2(g)![]() 2N2(g)��6H2O(g)����H1����541��8 kJ��mol��1����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g)
2N2(g)��6H2O(g)����H1����541��8 kJ��mol��1����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g)![]() N2(g)��2H2O(g)����H2����534 kJ��mol��1����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ__________________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��________(��K1��K2��ʾ)��
N2(g)��2H2O(g)����H2����534 kJ��mol��1����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ__________________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��________(��K1��K2��ʾ)��
(2)����2NO(g)��2CO(g)![]() N2(g)��2CO2(g)����һ���¶��£���1 L�ĺ����ܱ������г���0��1 mol NO��0��3 mol CO����Ӧ��ʼ���С�
N2(g)��2CO2(g)����һ���¶��£���1 L�ĺ����ܱ������г���0��1 mol NO��0��3 mol CO����Ӧ��ʼ���С�
��������˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����______(����ĸ����)��
A��c(CO)��c(CO2)
B�������л��������ܶȲ���
C��v(N2)����2v(NO)��
D�������л�������ƽ��Ħ����������
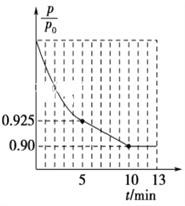
��ͼ1Ϊ�����ڵ�ѹǿ(p)����ʼѹǿ(p0)�ı�ֵ![]() ��ʱ��(t)�ı仯���ߡ�0��5min�ڣ��÷�Ӧ��ƽ����Ӧ����v(N2)��________��ƽ��ʱNO��ת����Ϊ________��
��ʱ��(t)�ı仯���ߡ�0��5min�ڣ��÷�Ӧ��ƽ����Ӧ����v(N2)��________��ƽ��ʱNO��ת����Ϊ________��
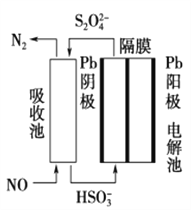
(3)ʹ�ü�ӵ绯ѧ���ɴ���ȼ�������е�NO��װ����ͼ��ʾ����֪���ص�����������Һ��pH��4��7֮�䣬д�������ĵ缫��Ӧʽ��____________________�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ��____________________________________________��
���𰸡� 4NH3(g)��O2(g)2N2H4(g)��2H2O(g)��H����526.2 kJ��mol��1 K1/K22 D 0��006 mol��L��1��min��1 80% 2HSO3-��2e����2H��===S2O42-��2H2O 2NO��2S2O42-��2H2O===N2��4HSO3-
��������(1)��4NH3(g)��3O2(g) ![]() 2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1����N2H4(g)��O2(g)
2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1����N2H4(g)��O2(g) ![]() N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2�����ݸ�˹���ɣ�����-����2�ã�4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=(��541.8kJ/mol)-(��534kJ/mol)��2=��526.2kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=
N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2�����ݸ�˹���ɣ�����-����2�ã�4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=(��541.8kJ/mol)-(��534kJ/mol)��2=��526.2kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=![]() ���ʴ�Ϊ��4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol��
���ʴ�Ϊ��4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol�� ![]() ��
��
(2)����2NO(g)��2CO(g) ![]() N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
��A��c(CO)=c(CO2)������ʾŨ�ȱ仯�������ж��Ƿ�Ϊƽ��״̬����A����B����Ӧ��������������䣬������䣬�����л��������ܶ�ʼ�ղ��䣬�����ж��Ƿ�Ϊƽ��״̬����B����C��v(N2)��=2v(NO)����ʾ��Ӧ����2v(N2)��=v(NO)�����ű�ʾ���淴Ӧ������ȣ���C����D���÷�Ӧ������������ʵ��������仯�ķ�Ӧ�������л�������ƽ��Ħ����������ʱ��ʾ��������ʵ������䣬 ˵����ƽ��״̬����D��ȷ����ѡD��
�����������ڵ�ѹǿ(P)����ʼѹǿ(P0)�ı�ֵ(P/P0)��ʱ��(t)�ı仯���ߣ�0��5min�ڣ�![]() =0.925�����ݰ���٤�����ɼ������ۣ�
=0.925�����ݰ���٤�����ɼ������ۣ�![]() =0.925��ƽ��ʱ
=0.925��ƽ��ʱ![]() =0.90��
=0.90��
2NO(g)�� 2CO(g) ![]() N2(g)��2CO2(g)
N2(g)��2CO2(g)
��ʼ(mol) 0.1 0.3 0 0
��Ӧ 2x 2x x 2x
5min��ƽ�� 0.1-2x 0.3-2x x 2x
5minʱ��![]() =0.925�����x=0.03mol��v(N2)=
=0.925�����x=0.03mol��v(N2)=![]() = 0.006mol��L��1��min��1��ƽ��ʱ��
= 0.006mol��L��1��min��1��ƽ��ʱ��![]() =0.90�����x=0.04mol��NO��ת����=
=0.90�����x=0.04mol��NO��ת����=![]() ��100%=80%���ʴ�Ϊ��0.006 mol��L��1��min��1��80%��
��100%=80%���ʴ�Ϊ��0.006 mol��L��1��min��1��80%��
(3)����������ԭ��Ӧ����������������ӣ��õ��ӣ����������������ӣ��缫��ӦʽΪ��2HSO3-+2e-+2H+�TS2O42-+2H2O����������������һ����������������ԭ��Ӧ�����ɵ��������ӷ�Ӧ����ʽΪ��2NO+2S2O42-+2H2O�TN2+4HSO3-���ʴ�Ϊ��2HSO3-+2H++2e-=S2O42-+2H2O��2NO+2S2O42-+2H2O=N2+4HSO3-��
�����͡������
��������
10
����Ŀ������ӵ����Ŀǰ������߱������Ķ��ε�ء�LiFePO4�ɼ���ظ��Ƶ����ϵ�İ�ȫ���ܣ��Ҿ�����Դ�ḻ��ѭ���������������Ѻõ��ص㣬������ӵ���������ϵ�����ѡ������LiFePO4��һ�ֹ���������ͼ��
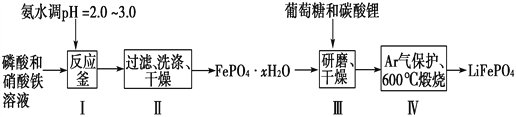
��֪��Ksp(FePO4��xH2O)��1.0��10��15��Ksp[Fe(OH)3]��4.0��10��38��
(1)�ںϳ�������ʱ���������pH�Ŀ����ǹؼ������pH<1.9��Fe3����������ȫ��Ӱ����������pH��3.0������ܴ��ڵ�������________________��
(2)������У�ϴ����Ϊ�˳�ȥFePO4��xH2O���渽�ŵ�________�����ӡ�
(3)ȡ3��FePO4��xH2O��Ʒ���������³�����ղ���ᾧˮ������ʵ���������±���
ʵ����� | 1 | 2 | 3 |
����ʧ���������� | 19.9% | 20.1% | 20.0% |
����ʧ������������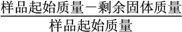 ��100%����x��_______(��ȷ��0.1)��
��100%����x��_______(��ȷ��0.1)��
(4)���������ĥ��������__________________________________��
(5)�ڲ������������LiFePO4��CO2��H2O�����������뻹ԭ�������ʵ���֮��Ϊ________��
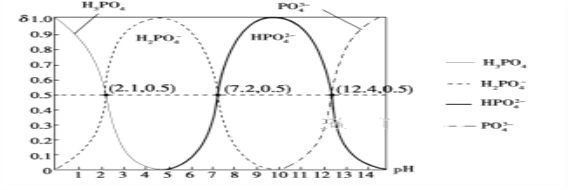
(6)H3PO4����Ԫ�ᣬ��ͼ�dz�������Һ�к����������ʵ�������(��)��pH�仯ʾ��ͼ����PO![]() ��һ��ˮ���ˮ�ⳣ��K1�ı���ʽΪ______��K1����ֵ��ӽ�______(����ĸ)��
��һ��ˮ���ˮ�ⳣ��K1�ı���ʽΪ______��K1����ֵ��ӽ�______(����ĸ)��
A��10��12.4����B��10��1.6 C��10��7.2 D��10��4.2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25��ʱ����pHΪa�������pHΪb��NaOH��Һ��ȡVaL�����ᣬͬ��NaOH��Һ�кͣ���VbLNaOH��Һ����գ�
��1����a+b=14����Va:Vb=_________�������֣���
��2����a+b=13����Va:Vb=_________�������֣���
��3����a+b>14����Va:Vb=_________�������ʽ������Va__________Vb���>��<��=��������a��6��b��8����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���l0mL0.1mol/L��HR��Һ������0.lmol/L��NH3��H2O ��Һ��������ҺpH�������Ա仯��ͼ�����з�������ȷ����

A. ab�㵼��������ǿ��˵��HRΪ����
B. b����Һ��c(NH3H2O)=c(R-)+c(H+)-c(OH-)
C. c����Һ������c(NH4+)>c(R-)>c(OH-)>c(H+)
D. �����£�HR��NH3H2O�ĵ���ƽ�ⳣ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�Һϳɳ���һ���»�����(��ͼ��ʾ)������W��X��Y��ZΪͬһ������Ԫ�أ�Z����������������X�����������һ�롣����������ȷ���ǣ� ��

A.WZ��ˮ��Һ�ʼ���
B.Ԫ�طǽ����Ե�˳��ΪX��Y��Z
C.Y������������ˮ������ǿ��
D.���»�������Y����8�����ȶ��ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��6g̼����ȫȼ�����ò����У�CO��CO2�������Ϊ2��3���ң�C(s)+O2(g)=CO(g) ��H=-110.35kJ��mol-1 CO(g)+O2(g)=CO2(g) ��H=-282.57 kJ��mol-1����6g̼��ȫȼ����ȣ���ʧ������Ϊ�� ��
A.56.51kJB.110.35kJC.196.46kJD.282.57kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������Ҫ��ҽ���м��壬��ͼ������������L�ĺϳ�·�ߡ�
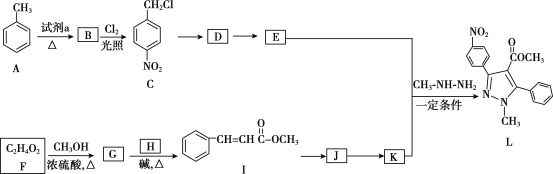
��֪��R1��CHO+R2CH2��COOR3![]()
R1��CHO+R2NH2![]() R1��CH=N��R2
R1��CH=N��R2
��1���Լ�a��__��
��2��C����D�ķ�Ӧ������__��
��3��D����E�Ļ�ѧ����ʽ��___��
��4������G�Ļ�ѧ����ʽ��__��
��5��H�Ľṹ��ʽ��__��
��6��д����������������I��ͬ���칹��Ľṹ��ʽ__��
a.�Ƿ�ʽ�ṹ
b.�ܷ���������Ӧ
c.�����ϵ�һ�ȴ�����2��
d.1mol���л�������2mol�������Ʒ�Ӧ
��7��K�ķ���ʽ��C10H8O2��K�Ľṹ��ʽ��__��
��8����2-����ϩ������Ϊԭ�ϣ�ѡ�ñ�Ҫ�����Լ����ϳ�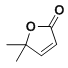 ��д���ϳ�·��__���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ������
��д���ϳ�·��__���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ʱ������
ʱ������![]() ����ˮ��Һ��ϵ�У�
����ˮ��Һ��ϵ�У�![]() ��
��![]() ��
��![]() �����и�����ռ�����ʵ�������
�����и�����ռ�����ʵ�������![]() ����ҺpH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
����ҺpH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
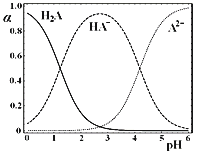
A.�ں�![]() A��
A��![]() ��
��![]() ����Һ�У���������NaOH���壬
����Һ�У���������NaOH���壬![]() һ������
һ������
B.�������ʵ�����NaHA��![]() ���������ˮ��������Һ��
���������ˮ��������Һ��![]()
C.NaHA��Һ�У�![]() ��ˮ����������
��ˮ����������![]() �ĵ�������
�ĵ�������
D.�ں�![]() A��
A��![]() ��
��![]() ����Һ�У���
����Һ�У���![]() ����
����![]() ��
��![]() ��һ�����
��һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1��2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18g��cm-3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������顣���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ�������Ʊ���ϩ���Թ�d��װ��Һ�壨���渲������ˮ���������й�˵������ȷ���ǣ� ��
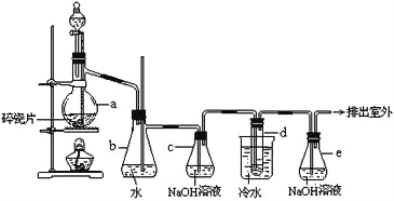
A.ʵ����Ϊ�˷�ֹ�л�������ӷ���ӦѸ�����߷�Ӧ�¶���170��
B.װ��c��װ��e�ж�ʢ��NaOH��Һ�������յ����ʲ���ͬ
C.�Ʊ���ϩ������1��2����������ķ�Ӧ���ͷֱ�����ȥ��Ӧ�ͼӳɷ�Ӧ
D.��d�в�����뱥��Na2CO3��Һ���ã����÷�Һ�ķ������з���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com