
����Ŀ����. ����п��һ�����뵼��,Ҳ����Ϊ�����ѧ����,�۵���1 520 �档
��1����̬пԭ�ӵļ۵����Ų�ʽ��_______��
��2������Ԫ��������,�縺��Se____S,��һ������Se____As(����>������<��)��
��3��H2Se�ķ��ӹ�����____,������ԭ�ӵ��ӻ����������____��
��4��H2O�ķе����H2Se�ķе�(-42 ��),��ԭ����________________��
��5������ZnΪ�������ܶѻ�,����λ����____��
�� �����仯��������������������ϢϢ��ء��ش���������:
��1��C��N��O����Ԫ�ص�һ�����ܴӴ�С��˳����________��
��2��1 mol N2F2����____ mol ������
��3��NH4BF4(�������)�Ǻϳɵ��������ܵ�ԭ��֮һ��1 mol NH4BF4��____ mol��λ����
��4����ȫ���Ҵ�ʱ�����Ļ�ѧ��ӦΪ10NaN3+2KNO3 = K2O+5Na2O+16N2����
��д����N2��Ϊ�ȵ�����ķ���________��
��Na2O�ľ����ṹ��ͼ��ʾ,�����߳�Ϊ566 pm,��������ԭ�ӵ���λ��Ϊ____,Na2O������ܶ�Ϊ______(ֻҪ������ʽ,���ؼ�������)��Na+ ��O2�������̾���Ϊ_____pm��

���𰸡� 3d104s2 < < V�� sp3 ˮ���Ӽ���������H2Se���Ӽ������ 12 N>O>C 3 2 CO 8 ![]() 245
245
����������. ��1��Zn��30��Ԫ�أ���ԭ�Ӻ�����30�����ӣ���3d��4s����Ϊ��۵��ӣ���۵����Ų�ʽΪ3d104s2����2��ͬһ����Ԫ�أ�Ԫ�ص縺������ԭ�������������С�����Ե縺��Se��S��ͬһ����Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ������Se��As����3��H2Se�۲���ӶԸ�����4�Һ���2���µ��Ӷԣ����ݼ۲���ӶԻ��������жϸ÷��ӿռ乹�ͼ�Seԭ���ӻ���ʽ�ֱ�ΪV�Ρ�sp3����4������������⻯���۷е�ϸߣ�H2O���������H2Se�������������H2O�ķе㣨100��������H2Se�ķе㣨-42�棩����5��п���ʾ������������ܶѻ���ԭ�Ӱ���ABABAB����ʽ�ѻ���������Znԭ�ӵ���λ��Ϊ12��
��. ��1��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���Ԫ��2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ�����ܣ�N��O��C����2��N2F2���ӽṹʽΪF-N=N-F�����l mol N2F2����3mol��������3��NH4BF4 ��笠������к���1����λ����Bԭ����F֮���γ�1����λ����l mol NH4BF4����2mol��λ������4����ԭ�����ͼ۵������ֱ���ȵĻ�Ϊ�ȵ����壬���뵪����Ϊ�ȵ�����ķ���ΪCO���ھ����а�ɫ����ĿΪ8��1/8+6��1/2=4����ɫ����ĿΪ8��Naԭ������ԭ����Ŀ֮��Ϊ2��1�����ɫ��Ϊ��ԭ�ӡ���ɫ��ΪNaԭ�ӣ���ɫ����λ��Ϊ4�����ɫ����λ��Ϊ8����������Ϊ4��62/6.02��1023g�����ܶ�Ϊ��4��62/6.02��1023g���£�566��10-10 cm��3=![]() ��Na+��O2�������̾���Ϊ��Խ��ߵ�1/4����Ϊ
��Na+��O2�������̾���Ϊ��Խ��ߵ�1/4����Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��M2+ ���������Ӳ㣬��M�����ڱ��е�λ���� �� ��
A. �ڶ��������� B. �ڶ�����IIA��
C. �ڶ�����VIIA�� D. ��������IIA��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ������������ԭ�������͵���
A. SO2����SO3��������Ҫʹ�ô���2SO2(g)+O2(g)![]() 2SO3(g)
2SO3(g)
B. 500 �����ҵ��¶ȱ����¸������ںϳɵ���ӦN2(g)+ 3H2 (g)![]() 2NH3(g) ��H<0
2NH3(g) ��H<0
C. H2��I2��HIƽ���������ѹ����ɫ����H2(g)+ I2(g)![]() 2HI(g)
2HI(g)
D. ����ʹ��ˮ���� Cl2��H2O![]() H����Cl����HClO
H����Cl����HClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ݻ�������ܱ������г���һ����A��B���������·�Ӧ��xA(g)��2B(s) ![]() yC(g)����H<0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
yC(g)����H<0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺

��1����A��Ũ�ȱ仯��ʾ�÷�Ӧ��0��10 min�ڵ�ƽ����Ӧ����v(A)��______________________��
��2������ͼʾ��ȷ��x��y��________��
��3��0��10 min������ѹǿ________(���������䡱��С��)��
��4���Ʋ��10 min�������߱仯�ķ�Ӧ����������______________________����16min�������߱仯�ķ�Ӧ����������________________________��
�ټ�ѹ��������A��Ũ�ȡ� ������C������ �����¢ݽ��¡� �Ӵ���
��5����ƽ����ƽ�ⳣ��ΪK1��ƽ���ƽ�ⳣ��ΪK2����K1________K2(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��Ҫ����0.2mol��L��1������������Һ450mL����������ƽ��ȡ����ʱ����ƽ����(���뼰����)���� ��
A. ����4.0gB. ����3.60gC. ����4.0gD. ����0.36g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������CrO3�������ڽ����Ƹ�����ҵ���������������Ļ�ѧ����ʽΪ��X��H2SO4��2CrO3��Na2SO4��H2O������X�Ļ�ѧʽ��
A. Na2CrO4 B. Na2Cr2O7 C. Na2Cr2O4 D. Na2CrO7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ͽ���CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶�/�� | |
500 | 800 | ||
��2H2(g)��CO(g) | K1 | 2.5 | 0.15 |
��H2(g)��CO2(g) | K2 | 1.0 | 2.50 |
��3H2(g)��CO2(g) | K3 | ||
��1���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3��________����K1��K2��ʾ����500 ��ʱ��÷�Ӧ����ijʱ�̣�H2(g)��CO2(g)��CH3OH(g)��H2O(g)��Ũ��(mol��L��1)�ֱ�Ϊ0.8��0.1��0.3��0.15�����ʱv��________v��(�>������������<��)��
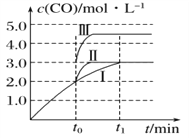
��2����3 L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c(CO)����Ӧʱ��t�仯���ߢ���ͼ��ʾ������t0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ����ߢ��Ϊ���ߢ�ʱ���ı��������_______________�������ߢ��Ϊ���ߢ�ʱ���ı��������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵����ȷ����
A. ��״���£�6. 72 L NO2��ˮ��Ӧת�Ƶĵ�����Ϊ0.1NA
B. 1 L 0.1 mol/L��̼������Һ�е���������������0.1NA
C. 1 mol Cl2������ Fe��Ӧת�Ƶ�����һ��Ϊ3NA
D. 1 mol Na2O��Na2O2�����������������������3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ��2NO2![]() 2NO+O2���ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
2NO+O2���ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
�ٵ�λʱ��������n molO2��ͬʱ����2n molNO2���ڵ�λʱ��������n molO2 ��ͬʱ������2n mol NO������NO2��NO��O2 �����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ2 : 2 : 1��״̬���ܻ���������ɫ���ٸı��״̬���ݻ��������ܶȲ��ٸı��״̬���� ��������ƽ����Է����������ٸı��״̬
A. �٢ܢ� B. �ڢۢ� C. �٢ۢ� D. �٢ڢۢܢݢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com