
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЖМЪЧЖЬжмЦкдЊЫиЃЌдзгАыОЖD>C>A>BЃЌЦфжаAЁЂBДІдкЭЌвЛжмЦкЃЌAЁЂCДІдкЭЌвЛжїзхЁЃCдзгКЫФкжЪзгЪ§ЕШгкAЁЂBдзгКЫФкжЪзгЪ§жЎКЭЃЌCдзгзюЭтВуЩЯЕФЕчзгЪ§ЪЧDдзгзюЭтВуЕчзгЪ§ЕФ4БЖЁЃЪдЛиД№ЃК
ЃЈ1ЃЉетЫФжждЊЫиЗжБ№ЪЧЃКA____ЃЌB___ЃЌC___ЃЌD____ЁЃ
ЃЈ2ЃЉетЫФжждЊЫижадкГЃЮТГЃбЙЯТЕФвКЬЌЛђЦјЬЌЧтЛЏЮяЕФЮШЖЈадгЩДѓЖјаЁЕФЫГађЪЧ_____ЁЃ
ЃЈ3ЃЉAгыBаЮГЩЕФШ§дзгЗжзгЕФЕчзгЪНЪЧ___ЃЌBгыDаЮГЩдзгИіЪ§БШЮЊ1ЁУ1ЕФЛЏКЯЮяЕФЕчзгЪНЪЧ_____ЁЃ
ЃЈ4ЃЉAдЊЫиФГбѕЛЏЮягыDдЊЫиФГбѕЛЏЮяЗДгІЩњГЩЕЅжЪЕФЛЏбЇЗНГЬЪНЪЧ_______ЁЃ
ЁОД№АИЁП(1)ЬМ бѕ Йш ФЦ ЃЈИї1ЗжЃЉ (2)H2O>CH4>SiH4 (2Зж)
(3)![]() ЃЈИї1ЗжЃЉ
ЃЈИї1ЗжЃЉ
(4)2CO2ЃЋ2Na2O2ЃН2Na2CO3ЃЋO2 (2Зж)
ЁОНтЮіЁП
ЪдЬтAЁЂBЁЂCЁЂDЖМЪЧЖЬжмЦкдЊЫиЃЌдзгАыОЖDЃОCЃОAЃОBЃЌЦфжаAЁЂBДІдкЭЌвЛжмЦкЃЌAЁЂCДІдкЭЌвЛжїзхЃЌЫФжждЊЫидкжмЦкБэжаЕФДѓжТЯрЖдЮЛжУЮЊЃК![]() ЃЌAЁЂCДІдкЭЌвЛжїзхЃЌЖўепжЪзгЪ§ЯрВю8ЃЌCдзгКЫФкжЪзгЪ§ЕШгкAЁЂBдзгКЫФкжЪзгЪ§жЎКЭЃЌЙЪBЕФжЪзгЪ§ЮЊ8ЃЌBЮЊOдЊЫиЃЌCдзгзюЭтВуЩЯЕФЕчзгЪ§ЪЧDдзгзюЭтВуЕчзгЪ§ЕФ4БЖЃЌЙЪCзюЭтВуЕчзгЪ§ЮЊ4ЃЌDЕФзюЭтВуЕчзгЪ§ЮЊ1ЃЌЙЪAЮЊCдЊЫиЃЌCЮЊSiдЊЫиЃЌDЮЊNaдЊЫиЃЌдђ
ЃЌAЁЂCДІдкЭЌвЛжїзхЃЌЖўепжЪзгЪ§ЯрВю8ЃЌCдзгКЫФкжЪзгЪ§ЕШгкAЁЂBдзгКЫФкжЪзгЪ§жЎКЭЃЌЙЪBЕФжЪзгЪ§ЮЊ8ЃЌBЮЊOдЊЫиЃЌCдзгзюЭтВуЩЯЕФЕчзгЪ§ЪЧDдзгзюЭтВуЕчзгЪ§ЕФ4БЖЃЌЙЪCзюЭтВуЕчзгЪ§ЮЊ4ЃЌDЕФзюЭтВуЕчзгЪ§ЮЊ1ЃЌЙЪAЮЊCдЊЫиЃЌCЮЊSiдЊЫиЃЌDЮЊNaдЊЫиЃЌдђ
ЃЈ1ЃЉИљОнвдЩЯЗжЮіПЩжЊЫФжждЊЫиУћГЦЗжБ№ЮЊЬМЁЂбѕЁЂЙшЁЂФЦЁЃ
ЃЈ2ЃЉЗЧН№ЪєаддНЧПЃЌЧтЛЏЮяЕФЮШЖЈаддНЧПЃЌЗЧН№ЪєадЪЧO>C>SiЃЌЫљвдЧтЛЏЮяЕФЮШЖЈадгЩДѓЖјаЁЕФЫГађЪЧH2O>CH4>SiH4ЁЃ
ЃЈ3ЃЉЬМгыбѕаЮГЩЕФШ§дзгЗжзгЪЧЖўбѕЛЏЬМЃЌЦфЕчзгЪНЪЧ![]() ЃЛФЦгыбѕаЮГЩЕФдзгИіЪ§БШЮЊ1ЁУ1ЕФЛЏКЯЮяЪЧЙ§бѕЛЏФЦЃЌЦфЕчзгЪНЪЧ
ЃЛФЦгыбѕаЮГЩЕФдзгИіЪ§БШЮЊ1ЁУ1ЕФЛЏКЯЮяЪЧЙ§бѕЛЏФЦЃЌЦфЕчзгЪНЪЧ![]() ЁЃ
ЁЃ
ЃЈ4ЃЉЖўбѕЛЏЬМФмгыЙ§бѕЛЏФЦЗДгІЩњГЩбѕЦјКЭЬМЫсФЦЃЌЗДгІЕФЗНГЬЪНЮЊ2CO2ЃЋ2Na2O2ЃН2Na2CO3ЃЋO2ЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗДгІFeЃЈsЃЉ+CO2ЃЈgЃЉ![]() FeOЃЈsЃЉ+COЃЈgЃЉЕФЦНКтГЃЪ§ЮЊK1ЃЛЗДгІFeЃЈsЃЉ+H2OЃЈgЃЉ
FeOЃЈsЃЉ+COЃЈgЃЉЕФЦНКтГЃЪ§ЮЊK1ЃЛЗДгІFeЃЈsЃЉ+H2OЃЈgЃЉ![]() FeOЃЈsЃЉ+H2ЃЈgЃЉЕФЦНКтГЃЪ§ЮЊK2ЁЃдкВЛЭЌЮТЖШЪБK1ЁЂK2ЕФжЕШчЯТБэЃК
FeOЃЈsЃЉ+H2ЃЈgЃЉЕФЦНКтГЃЪ§ЮЊK2ЁЃдкВЛЭЌЮТЖШЪБK1ЁЂK2ЕФжЕШчЯТБэЃК
ЮТЖШЃЈОјЖдЮТЖШЃЉ | K1 | K2 |
973 | 1.47 | 2.38 |
1173 | 2.15 | 1.67 |
ЃЈ1ЃЉМЦЫуЮТЖШЮЊ973KЪБЃЌЗДгІCO2ЃЈgЃЉ+H2ЃЈgЃЉ![]() COЃЈgЃЉ+H2OЃЈgЃЉ K=__________ЃЛ
COЃЈgЃЉ+H2OЃЈgЃЉ K=__________ЃЛ
ЃЈ2ЃЉФПЧАЙЄвЕЩЯгавЛжжЗНЗЈЪЧгУCO2РДЩњВњМзДМЃКCO2(g)ЃЋ3H2(g) ![]() CH3OH(g)ЃЋH2O(g)ЃЌдкЬхЛ§ЮЊ1 LЕФКуШнУмБеШнЦїжаЃЌГфШы1 mol CO2КЭ3 mol H2НјааЗДгІЁЃ
CH3OH(g)ЃЋH2O(g)ЃЌдкЬхЛ§ЮЊ1 LЕФКуШнУмБеШнЦїжаЃЌГфШы1 mol CO2КЭ3 mol H2НјааЗДгІЁЃ
ЂйИУЗДгІФмЙЛздЗЂНјааЕФдвђЪЧ________ЁЃ
ЂкЯТСаДыЪЉжаФмЪЙc(CH3OH)діДѓЕФЪЧ________ЁЃ
AЃЎНЕЕЭЮТЖШ
BЃЎГфШыHe(g)ЃЌЪЙЬхЯЕбЙЧПдіДѓ
CЃЎНЋH2O(g)ДгЬхЯЕжаЗжРыГіРД
DЃЎдйГфШы1 mol CO2КЭ3 mol H2
ЂлдкЮТЖШT1ЪБЃЌЕБЗДгІДяЕНЦНКтЪБЃЌВтЕУn(H2)ЃН2.4 molЃЛЦфЫћЬѕМўВЛБфЃЌдкЮТЖШT2ЪБЃЌЕБЗДгІДяЕНЦНКтЪБЃЌВтЕУn(CO2)ЃН0.82 molЃЌдђT2________T1(ЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁАЃНЁБ)ЁЃ
ЃЈ3ЃЉФГЪЕбщНЋвЛЖЈСПЕФCO2КЭH2ГфШывЛЖЈЬхЛ§ЕФУмБеШнЦїжаЃЌдкСНжжВЛЭЌЬѕМўЯТЗЂЩњЗДгІЃКCO2(g) ЃЋ3H2(g) ![]() CH3OH(g)ЃЋH2O(g) ІЄHЃНЃ49.0 kJЁЄmolЃ1ЁЃВтЕУCH3OHЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЯТЭМЫљЪОЃЌЛиД№ЮЪЬтЃК
CH3OH(g)ЃЋH2O(g) ІЄHЃНЃ49.0 kJЁЄmolЃ1ЁЃВтЕУCH3OHЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЯТЭМЫљЪОЃЌЛиД№ЮЪЬтЃК

ЂйЧњЯпIЁЂЂђЖдгІЕФЦНКтГЃЪ§ДѓаЁЙиЯЕЮЊKЂё________KЂђ(ЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁАЃНЁБ)ЁЃ
ЂквЛЖЈЮТЖШЯТЃЌдкШнЛ§ЯрЭЌЧвЙЬЖЈЕФСНИіУмБеШнЦїжаЃЌАДШчЯТЗНЪНЭЖШыЗДгІЮяЃЌвЛЖЮЪБМфКѓДяЕНЦНКтЁЃ
ШнЦї | Мз | вв |
ЗДгІЮя ЭЖШыСП | 1 mol CO2ЁЂ3 mol H2 | a mol CO2ЁЂb mol H2ЁЂ c mol CH3OH(g)ЁЂc mol H2O(g) |
ШєМзжаЦНКтКѓЦјЬхЕФбЙЧПЮЊПЊЪМЪБЕФ0.8БЖЃЌвЊЪЙЦНКтКѓввгыМзжаЯрЭЌзщЗжЕФХЈЖШЯрЕШЃЌЧвЦ№ЪМЪБЮЌГжЗДгІФцЯђНјааЃЌдђcЕФШЁжЕЗЖЮЇЮЊ________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЬМе§Рызг[Р§ШчЃЌCH3+ЃЌCH5+ЃЌ(CH3)3C+ЕШ]ЪЧгаЛњЗДгІжаживЊЕФжаМфЬхЁЃХЗРЃЈGЃЎOlahЃЉвђдкДЫСьгђбаОПжаЕФзПдНГЩОЭЖјШйЛё1994ФъХЕБДЖћЛЏбЇНБЁЃЬМе§РызгCH5+ПЩвдЭЈЙ§CH4дкЁАГЌЧПЫсЁБжадйЛёЕУвЛИіH+ЖјЕУЕНЃЌЖјCH5+ЪЇШЅH2ПЩЕУCH3+ЁЃ
ЃЈ1ЃЉCH3+ ЪЧЗДгІадКмЧПЕФе§РызгЃЌЪЧШБЕчзгЕФЃЌЦфЕчзгЪНЪЧ__________________ЁЃ
ЃЈ2ЃЉCH3+ жа4ИідзгЪЧЙВЦНУцЕФЃЌШ§ИіМќНЧЯрЕШЃЌМќНЧгІЪЧ_________ЃЈЬюНЧЖШЃЉЁЃ
ЃЈ3ЃЉ(CH3)2CH+ дкNaOHЕФЫЎШмвКжаЗДгІНЋЕУЕНЕчжаадЕФгаЛњЗжзгЃЌЦфНсЙЙМђЪНЪЧ_____________ЁЃ
ЃЈ4ЃЉ(CH3)3C+ ШЅЕєH+ КѓНЋЩњГЩЕчжаадЕФгаЛњЗжзгЃЌЦфНсЙЙМђЪНЪЧ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖдПЩФцЗДгІ4NH3(g)ЃЋ5O2(g)![]() 4NO(g)ЃЋ6H2O(g)ЃЌЯТСаа№Ъіжае§ШЗЕФЪЧ
4NO(g)ЃЋ6H2O(g)ЃЌЯТСаа№Ъіжае§ШЗЕФЪЧ
A. ДяЕНЛЏбЇЦНКтЪБ4vе§(O2)ЃН5vФц(NO)
B. ШєЕЅЮЛЪБМфФкЩњГЩx mol NOЕФЭЌЪБЃЌЯћКФx mol NH3ЃЌдђЗДгІДяЕНЦНКтзДЬЌ
C. ДяЕНЛЏбЇЦНКтЪБЃЌШєдіДѓШнЦїЕФЬхЛ§ЃЌдђе§ЗДгІЫйТЪМѕаЁЃЌФцЗДгІЫйТЪдіДѓ
D. ЛЏбЇЗДгІЫйТЪЕФЙиЯЕЪЧ2vФц(NH3)ЃН3vе§(H2O)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙигкгаЛњЮяa(![]() )ЁЂb(
)ЁЂb(![]() )ЁЂc(
)ЁЂc(![]() )ЕФЫЕЗЈе§ШЗЕФЪЧ
)ЕФЫЕЗЈе§ШЗЕФЪЧ
A. aЁЂbЁЂcЕФЗжзгЪНОљЮЊC8H8
B. aЁЂbЁЂcОљФмгыфхЫЎЗЂЩњЗДгІ
C. aЁЂbЁЂcжажЛгаaЕФЫљгадзгЛсДІгкЭЌвЛЦНУц
D. aЁЂbЁЂcЕФвЛТШДњЮяжаЃЌbга1жжЃЌaЁЂcОљга5жж(ВЛПМТЧСЂЬхвьЙЙ)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЪЕбщаЁзщгУЯТСазАжУНјааввДМДпЛЏбѕЛЏЕФЪЕбщЁЃ

ЃЈ1ЃЉЪЕбщЙ§ГЬжаЭЭјГіЯжКьЩЋКЭКкЩЋНЛЬцЕФЯжЯѓЃЌЧыаДГіЯргІЕФЛЏбЇЗДгІЗНГЬЪНЃК______________________________________________ЁЃ
дкВЛЖЯЙФШыПеЦјЕФЧщПіЯТЃЌЯЈУ№ОЦОЋЕЦЃЌЗДгІШдФмМЬајНјааЃЌЫЕУїИУввДМбѕЛЏЗДгІЪЧ________ЗДгІЁЃ
(2)МзКЭввСНИіЫЎдЁзїгУВЛЯрЭЌЁЃ
МзЕФзїгУЪЧ__________________________ЃЛввЕФзїгУЪЧ__________________________ЁЃ
ЃЈ3ЃЉЗДгІНјаавЛЖЮЪБМфКѓЃЌИЩдяЪдЙмaжаФмЪеМЏЕНВЛЭЌЕФЮяжЪЃЌЫќУЧЪЧ________________ЁЃМЏЦјЦПжаЪеМЏЕНЕФЦјЬхЕФжївЊГЩЗжЪЧ__________________________ЁЃ
ЃЈ4ЃЉШєЪдЙмaжаЪеМЏЕНЕФвКЬхгУзЯЩЋЪЏШяЪджНМьбщЃЌЪджНЯдКьЩЋЃЌЫЕУївКЬхжаЛЙКЌга__________ЁЃвЊГ§ШЅИУЮяжЪЃЌПЩЯШдкЛьКЯвКжаМгШы________________ЃЈЬюаДзжФИЃЉЁЃ
a.ТШЛЏФЦШмвК b.БН
c.ЬМЫсЧтФЦШмвК d.ЫФТШЛЏЬМ
ШЛКѓЃЌдйЭЈЙ§________________ЃЈЬюЪЕбщВйзїУћГЦЃЉМДПЩГ§ШЅЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЫЎШШЗЈжЦБИFe3O4ФЩУзПХСЃЕФзмЗДгІЮЊЃК3Fe2++2S2O32-+O2+XOH- =Fe3O4+S4O62-+2H2OЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A. СђдЊЫиБЛбѕЛЏЃЌЬњдЊЫиБЛЛЙд
B. Fe2+ЁЂS2O32- ЖМЪЧЛЙдМС
C. X=2
D. УПЩњГЩ1mol Fe3O4ЃЌдђзЊвЦЕчзгЪ§2mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAЁЂBЁЂEЮЊМвЭЅжаГЃМћгаЛњЛЏКЯЮяЃЌAгыИпУЬЫсМиЫсадШмвКЗДгІПЩЩњГЩBЃЌDЪЧЪЏгЭЛЏЙЄЗЂеЙЫЎЦНЕФБъжОЃЌEЪЧвЛжжГЃМћЕФИпЗжзгВФСЯЁЃИљОнЯТУцЕФзЊЛЏЙиЯЕЛиД№ЯТСаЮЪЬтЃК
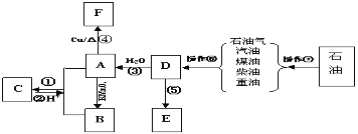
(1)DЕФНсЙЙМђЪНЮЊЃК_____________ЁЃ
(2)BжаЙйФмЭХЕФУћГЦЮЊЃК______________ЁЃ
(3)дкЂй~ЂнжаЪєгкбѕЛЏЗДгІЕФЪЧ_________ЃЛЪєгкМгГЩЗДгІЕФЪЧ________ЁЃ(ЬюађКХ)
(4)аДГіЯТСаЗДгІЗНГЬЪНЃКЗДгІЂй________________________ЃЛ
ЗДгІЂм_________________________ЁЃ
(5)гУAЁЂBжЦБИЮяжЪCЪБЃЌГ§ШЅCжаКЌгаЕФдгжЪбЁдёЕФЪдМСМАВйзїЗжБ№ЮЊ___________ЃЌ___________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЩщ(As)МАЦфЛЏКЯЮягыЩњВњЁЂЩњЛюУмЧаЯрЙиЃЌЙуЗКгІгУдкЩБГцМСвдМАвЉЮяжаЁЃЛиД№ЯТСаЮЪЬтЃК
(1)ЩщЪЧЕкЫФжмЦкVAзхдЊЫиЁЃЯТСаЙигкЕкVAзхдЊЫиМАЦфЛЏКЯЮяЕФЫЕЗЈВЛе§ШЗЕФЪЧ_______(ЬюађКХ)ЁЃ
aЃЎЗаЕуЃКPH3<NH3
bЃЎЫсадЃКHNO3>H3AsO4
cЃЎAsЕФбѕЛЏЮяЕФЫЎЛЏЮяЪЧЧПМю
dЃЎаЮГЩЕФМђЕЅРызгАыОЖЫцзХдзгађЪ§ЕндіЖјдіДѓ
(2)ЙЄвЕЩњВњжаГЃгУЁАЙХЪЯЪдЩщЗЈЁБМьбщЮЂСПЩщЃЌЦфЗДгІдРэЮЊЃК
ЂйНЋКЌЩщЛЏКЯЮязЊЛЏЮЊОпгаМЋЧПЛЙдадЕФAsH3ЃЛ
ЂкAsH3гыAgNO3ШмвКЗДгІВњЩњAs2O3гыСэвЛжжЙЬЬхЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊ_____________ЁЃ
(3)ЩщМАЦфЛЏКЯЮяМИКѕЖМгаЖОЃЌЭЈГЃ+3МлЩщЛЏКЯЮяЖОадЧПгк+5МлЩщЛЏКЯЮяЁЃКЃВњЦЗжаКЌгаЮЂСП+5МлЩщЛЏКЯЮяЃЌЪГгУКЃЯЪКѓВЛФмТэЩЯНјЪГЫЎЙћЕФдвђЪЧ________________________________ЁЃ
(4)ЩщЫс(H3AsO4)ПЩгУгкжЦдьЩБГцМСЁЂвЉЮяЁЃAs2O3ШмгкЯЁЯѕЫсжаПЩЕУЩщЫсЃЌДЫЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________________________________ЁЃ
(5)РћгУЕЅжЪЬњДІРэЫЎЬхЩщЮлШОЕФдРэЮЊЃКЕЅжЪЬњдкЫЎЬхжаБЛбѕЛЏИЏЪДЕУЕНЫЎКЯбѕЛЏЬњ[Fe(OH)3КЭFeOOH]ЃЌЮќИНГСНЕЩщЕФЛЏКЯЮяЁЃдкЦфЫќЬѕМўвЛЖЈЪБЃЌЕїНкЫЎбљЕФpHЃЌЕУЕНГ§ЩщаЇТЪЧњЯпШчЭМЫљЪОЁЃ
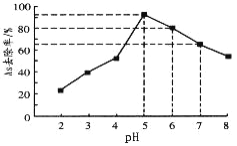
ЂйЧыНтЪЭЫЎбљЫсадНЯЧПЪБЃЌГ§ЩщаЇТЪНЯЕЭЕФдвђЪЧ_____________________ЁЃ
ЂкЙњМввћгУЫЎБъзМЙцЖЈЫЎжаЩщЕФКЌСПгІЕЭгк0.05mgЁЄL-1(1mg=1000ІЬg)ЁЃФГЫЎбљжаКЌЩщзмХЈЖШЮЊ100ІЬgЁЄL-1ЃЌШєПижЦЫЎбљЕФpH=6ЃЌГіЫЎКѓЕФЩщХЈЖШ_________________(ЬюЁАФмЁБЛђЁАЗёЁБ)ДяЕНвћгУЫЎБъзМЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com