��2010?���գ���������ΪLiCoO
2������ӵ���ѱ��㷺������Яʽ��Դ�����ܵ���Դ�ѷ����������һ����չ��
��1�����ʯ��LiFePO
4��һ��DZ�ڵ�����ӵ���������ϣ�������ͨ����NH
4��
2Fe��SO
4��
2��H
3PO
4��LiOH��Һ������������Ӧ�����ó�����80����ո�����³��Ͷ��Ƶã�
�ٹ�������ӦͶ��ʱ��������NH
4��
2Fe��SO
4��
2��LiOH��Һֱ�ӻ�ϵ�ԭ����
Fe2+�ڼ��������¸����ױ�����
Fe2+�ڼ��������¸����ױ�����
��
�ڹ�������Ӧ�Ļ�ѧ����ʽΪ
��NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O
��NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O
��
�۸��³���ǰ������LiFePO
4�м�����������̿�ڣ������ó��˿��Ը��Ƴ��ͺ��LiFePO
4�ĵ��������⣬����
�������O2��Ӧ����ֹLiFePO4�е�Fe2+������
�������O2��Ӧ����ֹLiFePO4�е�Fe2+������
��
��2���Ͼ�����ӵ�ص�����������������Ҫ����LiCoO
2������AI��Fe�ȣ���ͨ������ʵ�鷽�������ܡ�ﮣ�
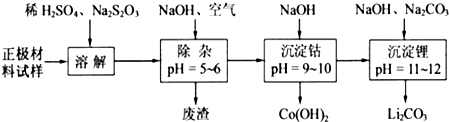
���������ܽ�����У�S
2O
32-��������SO
42-��LiCoO
2���ܽ�����з�Ӧ�Ļ�ѧ����ʽΪ
8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O
8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O
��
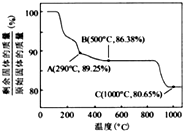
��Co��OH��
2�ڿ����м���ʱ��������������¶ȵı仯����ͼ��ʾ����֪�ܵ��������������290��ʱ����ȫ��ˮ����1000��ʱ��ʣ�����ijɷ�Ϊ
CoO
CoO
�����ѧʽ������350��400�淶Χ�ڣ�ʣ�����ijɷ�Ϊ
Co2O3��Co3O4
Co2O3��Co3O4
�����ѧʽ����





 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

 ��2010?���ն�ģ���������ٻ��š����ֵķз�����ķ���Prius����������϶������������õ綯������ȼ������߽���ƶ����֣��������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬��
��2010?���ն�ģ���������ٻ��š����ֵķз�����ķ���Prius����������϶������������õ綯������ȼ������߽���ƶ����֣��������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬��