
��������ʵ��������������õ�ʵ�������ȷ���� (����)
| ʵ����������� | ʵ����� | |
| A | ��2 mL 0.1 mol��L-1 CH3COOH��Һ�еμӵ�Ũ�ȵ�NaHCO3��Һ�������ݲ��� | ���ԣ� CH3COOH > H2CO3 |
| B | ��2 mL 0.1 mol��L-1 NaOH��Һ�еμ�3��0.1 mol��L-1 MgCl2��Һ�����ְ�ɫ�������ٵμ�3��0.1 mol��L-1 FeCl3��Һ�����ֺ��ɫ���� | ���ԣ� Mg(OH)2>Fe(OH)3 |
| C | ��������ˮ�Ҵ��м������Na�����ɿ����ڿ�����ȼ�յ����� | CH3CH2OH��������� |
| D | ��3 mLϡH2SO4��Һ������Zn��Ӧ��������ϡ��ʱ������1 mLŨH2SO4����Ѹ�ٲ����϶����� | H2SO4Ũ������Ӧ���ʼӿ� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ӽ��ܵ����������ڰ뵼�幤ҵ�͵����մɵ�������һ�ִ��Ⱥܸߵ�������乤ҵ��ȡ�������£�


��1��ʵ���ҹ������������������̨����Ȧ���ձ��� ��©����
 ��2������A�ijɷֳ�������Co3��PO4��3��Fe(OH)3��� ���ѧʽ��������CO3(PO4)2��Ŀ���� ��
��2������A�ijɷֳ�������Co3��PO4��3��Fe(OH)3��� ���ѧʽ��������CO3(PO4)2��Ŀ���� ��
��3��Co��ϡ���ᷴӦ���� Co2+�����ӷ���
ʽΪ�� ��
��4������B��������ϴ�ӡ�����������գ������ط���ͼ���ң�д�����л�ѧ��Ӧ��
��ʽ��
��AB�� ��
��BC�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
�����������ʻ��ã�����������Ϻ�����������ը
��ʵ������ȡ����ʱ��Ϊ�˷�ֹ������Ⱦ���������������������������Һ����
��������ˮ��������ǿ�ھ�����ˮ
�ܼ���HCl�������Ƿ����Cl2�����ǽ�����ͨ����������Һ
�ݳ�ȥHCl�����е�Cl2���ɽ�����ͨ�뱥��ʳ��ˮ
A���٢ڢۡ����� B���ڢۢ� C���� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵�����ʾ��ȷ���� (����)
A�������ʵ������������������ֱ���ȫȼ�գ����߷ų���������
B��A��B��Ϊͬ�������壬��Aת��ΪBʱ��H����119 kJ��mol��1��֪B��A�ȶ�
C���Ԧ�H �� 0�Ļ�ѧ��Ӧ������Ҫ���ȣ�һ���ܹ��Է����е���
D����101 kPaʱ��H2ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)��O2(g)===2H2O(l)����H����571.6
kJ��mol��1����H2��101 kPaʱ��ȼ����Ϊ571.6 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��CO(g)��H2O(g)  CO2(g)��H2(g) ∆H���C 41 kJ/mol
CO2(g)��H2(g) ∆H���C 41 kJ/mol
��ͬ�¶��£��������ͬ�����������ܱ������У�����һ�����ķ�Ӧ�����Ӧ��
����������£�
| ������� | ��ʼʱ���������ʵ���/mol | ��ƽ�������ϵ �����ı仯 | ||||
| CO | H2O | CO2 | H2 | |||
| �� | 1 | 4 | 0 | 0 | �ų�������32.8 kJ |
|
| �� | 0 | 0 | 1 | 4 | �����仯��Q |
|
����˵���У�����ȷ����
A���������з�Ӧ��ƽ��ʱ��CO��ת����Ϊ80%
B����������CO��ת���ʵ�����������CO2��ת����
C��ƽ��ʱ����������CO2��Ũ�����
D���������з�Ӧ���ʣ�v��CO��= v��H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڹ�����ռ����Ҫ��λ��
��1���ϳɰ���ҵ�У��ϳ�����ÿ����2 mol NH3���ų�92.2 kJ������
��ҵ�ϳɰ����Ȼ�ѧ����ʽ�� ��
��2����ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO(NH2)2]����Ӧ�Ļ�ѧ����ʽΪ��2NH3 (g)+ CO2 (g)  CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
| T / �� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
�٦�H���>������<����=���� 0��
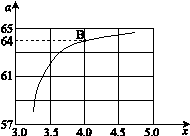
����һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ� ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ��������Ĺ�ϵ��������x����������ԭ���� ��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ��������Ĺ�ϵ��������x����������ԭ���� ��
�� ��ͼ�е�B�㴦��NH3��ƽ��ת����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͬ��������Ԫ�أ���ԭ����������������˵������������
A������������������ B��Ԫ����������ϼ۾���+1������+7
C��ԭ�Ӱ뾶��С D���������������ǽ���������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ǿ����������⣬�۷�����������Ⱦ�����Դ�����ʣ����������ƶ�������Ե�������ʩ���±���ij����ij�յĿ����������棺
| ��Ⱦָ�� | ��Ҫ��Ⱦ�� | ������������ | ��������״�� |
| 55 | SO2 | II | �� |
����ijУ�о���ѧϰС��Ա�����Ҫ��Ⱦ��SO2��������ij������������̽����
��1������ͼ��ʾװ�ý���ʵ�顣

��Aװ�õ�������____________________��
��ʵ������У�Bװ����ʯ����ֽ����ɫû�з����仯��Cװ����ʪ�����ɫʯ��
��ֽ���________________ɫ��˵��SO2��ˮ��Ӧ����һ���ᣬ�仯ѧ��Ӧ����ʽ�� ��
��Dװ�õ�������__________________________________________________��
D�з�����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
��2����ʢ��ˮ���ձ���ͨ��SO2���壬���������Һ��pH________7���>����=����<������Ȼ��ÿ��1h�ⶨ��pH������pH��_______������С������ ֱ���㶨��ԭ���� ��д����Ӧ�����ӷ���ʽ����
��3��SO2�γ��������һ;��Ϊ��SO2������е�O2��Ʈ���������·�Ӧ����SO3��SO3���ڽ�ˮ����H2SO4�� ���ڴ˹����е�Ʈ������Ϊ___________�����������������������
��4��SO2������е�������ˮ��Ӧ����������γ����ꡣ���п����׳������ꡣ
��5�������ŷŵ�β�������ᡢ���ʵȹ�ҵ�����ų��ķ����ж����е����������������������ˮ����ת��Ϊ__________________��������������һ��Ҫԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��DZˮͧ�п��ù���������Ϊ����������ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ������ͼ�е�ʵ��װ�ý���ʵ�飬֤�������� �ƿ�����������
�ƿ�����������

��A����ȡCO2��װ�á�д��A�з�����Ӧ�Ļ�ѧ����ʽ��______________________________________��
��B��ʢ�б���NaHCO3��Һ����������______________________________��
��д�����������������̼��Ӧ�Ļ�ѧ����ʽ__________________________
_______________________________________________________________��
��D��ʢ�ŵ��Լ���________����Ŀ����_____________________________��
���Թ�F���ռ����������һ��ʵ�������___________________________
________________________________________________________________��
(2)��ijѧ���ж�SO2��Na2O2��Ӧ�����������ƣ�����Ϊ�����жϺ�����____ ____����Ҫ˵�����ɣ�___________________________________________
____����Ҫ˵�����ɣ�___________________________________________
_________________________________________________________________��
�ڸ�ͬѧ���϶���Ӧ���Ƿ����������ɣ���ʹ����ͼ��ʾװ�ý���ʵ��(ͼ������̨��װ������ȥ)��

װ��B��������_______________ ___________________________________��
___________________________________��
D��������_______________________________________________________��
��Ϊȷ�Ϸ�Ӧ�����ͬѧ���������ʵ�鲽�裬����Ϊ�����IJ���˳����________(ѡ�����)��
A���ô����ǵ�ϸľ����������ܿ�a���۲�ϸľ���Ƿ�ȼ
B����Cװ���з�Ӧ��Ĺ���������������ˮ�����Һ
C������ɵ���Һ�м����������ữ�����ᱵ��Һ���۲��Ƿ��г�������
D������ɵ���Һ���ȼ������ᣬ�ټ����Ȼ�����Һ���۲��Ƿ������ݻ����
����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com