
����Ŀ��������ЧӦ����ȫ���ע�Ļ�������֮һ��C02��Ŀǰ�����к�����ߵ�һ���������壬C02���ۺ������ǽ�����Ҽ���Դ�������Ч;����
(1)�о�����C02��H2�ڴ��������¿ɷ�����Ӧ����CH3OH����֪���ַ�Ӧ���Ȼ�ѧ����ʽ���£�CH3OH��g��+![]() O2��g��= CO2��g��+2H2O��g����H1=akJmol-��H2��g��+
O2��g��= CO2��g��+2H2O��g����H1=akJmol-��H2��g��+![]() O2��g��=H2O��l����H2����CO2��g��+3H2��g��= CH3OH��g��+2H2O��l����H=___kJmol-��
O2��g��=H2O��l����H2����CO2��g��+3H2��g��= CH3OH��g��+2H2O��l����H=___kJmol-��
(2)C02������Ҳ�ܺϳɵ�̼ϩ����2C02(g)+6H2(g) =C2H4(g)+4H20(g)����ͬ�¶��� ƽ��ʱ��������̬���ʵ����ʵ�����ͼ��ʾ������c��ʾ������Ϊ____(�ѧʽ)��

(3)C02��H2�ڴ���Cu/ZnO�����¿ɷ�������ƽ�з�Ӧ���ֱ�����CH3OH��CO��
��ӦA: C02(g)+3H2(g) = CH30H(g)+H20(g)
��ӦB: C02(g)+H2(g)=C0(g)+H20(g)
����C02��H2��ʼͶ�ϱ�Ϊ1 : 3ʱ���¶ȶ�C02ƽ��ת���ʼ��״���CO���ʵ�Ӱ����ͼ��ʾ��
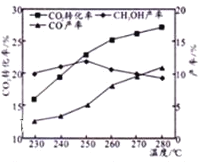
����ͼ��֪�¶�����CO�IJ�������������Ҫԭ�������________��
����ͼ��֪��ȡCH3OH�����˵��¶���____________�����д�ʩ�������C02ת��ΪCH3OH��ƽ��ת���ʵ���______(����ĸ)��
A.ʹ�ô��� B.������ϵѹǿ C.����C02�ͳ��ij�ʼͶ�ϱ�
(4)�ڴ�������ͨ��ʩ�ӵ�ѹ�ɽ��ܽ���������Һ�еĶ�����ֱ̼��ת��Ϊ�Ҵ��������� �Ҵ��ĵ缫��ӦʽΪ_________��
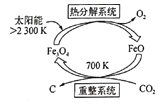
(5)��C02��ȡC��̫���ܹ�����ͼ��ʾ�����ȷֽ�ϵͳ�������ķ�ӦΪ 2Fe304 ![]() 6Fe0+02�������ֽ�1mo;Fe304ת�Ƶ��ӵ����ʵ���Ϊ______��������ϵͳ��������Ӧ�Ļ�ѧ����ʽΪ_______��
6Fe0+02�������ֽ�1mo;Fe304ת�Ƶ��ӵ����ʵ���Ϊ______��������ϵͳ��������Ӧ�Ļ�ѧ����ʽΪ_______��
���𰸡�3b��a C2H4 ��ӦB����Ӧ�����ȷ�Ӧ���¶�����ƽ�������ƶ���CO�������� 250�� AC 2CO2��12H����12e��=C2H5OH��3H2O 2 mol 6FeO+CO2![]() 2Fe3O4+C
2Fe3O4+C
��������
��֪��CH3OH(g)+![]() O2(g)�TCO2(g)+2H2O(1)��H1=akJmol-1����H2(g)+
O2(g)�TCO2(g)+2H2O(1)��H1=akJmol-1����H2(g)+![]() O2(g)=H2O(1)��H2=bkJmol-1�����ݸ�˹����֪������3-�ٵã�CO2(g)+3H2(g)CH3OH(g)+H2O(g)��
O2(g)=H2O(1)��H2=bkJmol-1�����ݸ�˹����֪������3-�ٵã�CO2(g)+3H2(g)CH3OH(g)+H2O(g)��
(2)����ͼ֪�������¶ȣ��������ʵ�������˵��ƽ�������ƶ���������Ӧ�Ƿ��ȷ�Ӧ��a���������¶����ߣ����ʵ�������Ϊ������̼��b��c�����¶����������ʵ������ͣ�Ϊ������ˮ����ϩ����ˮ�ı仯��������ϩ���ݴ��ж�c���ߴ������ʣ�
(3)�ٷ�ӦB����Ӧ�����ȷ�Ӧ���¶�����ƽ�������ƶ���CO�������ߣ�
�ھ�ͼʾ���з���250��״�ת������ߣ���CO2(g)+3H2(g)CH3OH(g)+H2O(g)��H1=-53.7kJmol-8 ��֪���CO2ת��ΪCH3OHƽ��ת���ʣ�Ӧʹƽ���������ƶ����ɽ����¶ȣ�����Ũ�ȣ�
(4)���ʱ��������̼��b���ϵõ��ӷ�����ԭ��Ӧ�����Ҵ���
(5)�÷�Ӧ��FeԪ�ػ��ϼ���+3�۱�Ϊ+2�ۣ�OԪ�ػ��ϼ���-2�۱�Ϊ0�ۣ�����ת�Ƶ��Ӻ�Fe3O4֮��Ĺ�ϵʽ���㣻����ͼ֪����Ӧ���Ƕ�����̼��FeO���������������������C����Ӧ������700K��
(1)��֪��CH3OH(g)+![]() O2(g)�TCO2(g)+2H2O(1)��H1=akJmol-1����H2(g)+
O2(g)�TCO2(g)+2H2O(1)��H1=akJmol-1����H2(g)+![]() O2(g)=H2O(1)��H2=bkJmol-1�����ݸ�˹����֪������3-�ٵã�CO2(g)+3H2(g)CH3OH(g)+H2O(g))������H=(3b-a)kJmol-1��
O2(g)=H2O(1)��H2=bkJmol-1�����ݸ�˹����֪������3-�ٵã�CO2(g)+3H2(g)CH3OH(g)+H2O(g))������H=(3b-a)kJmol-1��
(2)����ͼ֪�������¶ȣ��������ʵ�������˵��ƽ�������ƶ���������Ӧ�Ƿ��ȷ�Ӧ��a���������¶����ߣ����ʵ�������Ϊ������̼��b��c�����¶����������ʵ������ͣ�Ϊ������ˮ����ϩ����ˮ�ı仯��������ϩ������b���ߴ���H2O��c���ߴ�����ϩ��
(3)����ͼ2��֪�¶�����CO�IJ�������������Ҫԭ������Ƿ�ӦB����Ӧ�����ȷ�Ӧ���¶�����ƽ�������ƶ���CO�������ߣ�
(3)�ھ�ͼʾ���з���250��״�ת������ߣ��ʻ�ȡCH3OH�����˵��¶���250�棻
A��ʹ�ô�����ƽ�ⲻ�ƶ����������ת���ʣ���A����
B��������ϵѹǿ��ƽ�����������ƶ�����״�ת���ʣ���B��ȷ��
C������CO2��H2�ij�ʼͶ�ϱȣ�������������ת���ʣ�������̼��ת���ʼ�С����C����
�ʴ�ΪAC��
(4)���ʱ��������̼��b���ϵõ��ӷ�����ԭ��Ӧ����C2H5OH���缫��ӦʽΪ2CO2+12H++12e--=C2H5OH+3H2O��
(5)�÷�Ӧ��FeԪ�ػ��ϼ���+3�۱�Ϊ+2�ۣ�OԪ�ػ��ϼ���-2�۱�Ϊ0�ۣ��ֽ�2mol����������ת��4mol���ӣ���ֽ�1mol����������ת��2mol���ӣ�����ͼ֪����Ӧ���Ƕ�����̼��FeO���������������������C����Ӧ������700K����Ӧ����ʽΪ6FeO(S)+CO2![]() 2Fe3O4(S)+C��
2Fe3O4(S)+C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���AgNO3��Һ�ֱ�ζ�Ũ�Ⱦ�Ϊ0.01mol/L��KCl��K2C2O4��Һ�������ܽ�ƽ��ͼ����ͼ��ʾ(������C2O42-��ˮ��)������������ȷ���� ( )
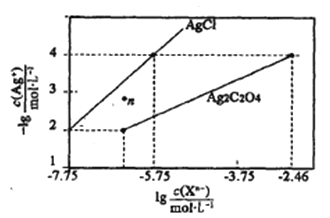
A.Ksp(Ag2C2O4)������������10-7
B.n���ʾAgCl�IJ�������Һ
C.��c (Cl-)=c(C2O42-)�Ļ��Һ�е���AgNO3��Һʱ��������Ag2C2O4����
D.Ag2C2O4 +2Cl�C(aq)2AgCl+C2O42-(aq)��ƽ�ⳣ��Ϊ109.04
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����̷���Ҫ���̷��к����ж��������谷(�ṹ��ͼ)��
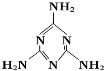
���й��������谷���ӵ�˵������ȷ���ǣ� ��
A. һ�������谷�����й�����15������
B. ���е�ԭ�Ӿ���ȡsp3�ӻ�
C. ���ڼ��Է���
D. �����谷������ͬʱ���м��Լ��ͷǼ��Լ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��AG ����Ϊ AG= lg![]() ������ AG ����ʾ��Һ����ȣ�˵������ȷ����
������ AG ����ʾ��Һ����ȣ�˵������ȷ����
A.��һ���¶��£���Һ������Խǿ��AG Խ��
B.65��ʱ��pH �� AG �Ļ��㹫ʽΪ AG = 2 (7 �C pH)
C.����Һ�Լ��ԣ��� AG < 0
D.����Һ�����ԣ��� AG = 0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ2NO2N2O4(g) H<0����һ������NO2����ע�����в��ܷ⣬�ı����λ�õĹ����У�����������ʱ��ı仯��ͼ��ʾ(������ɫԽ�����ԽС)������˵������ȷ����
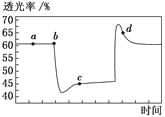
A.b ��IJ�����ѹ��ע����
B.c ����a����ȣ�c(NO2)����c(N2O4)��С
C.d �㣺��(��)<��(��)
D.����c�㽫�¶Ƚ��ͣ������ʽ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪±�����ڼ��������¿��Է�������ˮ�ⷴӦ��(R ��������)R��X+NaOH![]() R��OH+NaBr��A��B��C��D��E��F��G��H �����л�������������ת����ϵ�����ֻ�����ķ���ʽ�Ѹ�����
R��OH+NaBr��A��B��C��D��E��F��G��H �����л�������������ת����ϵ�����ֻ�����ķ���ʽ�Ѹ�����
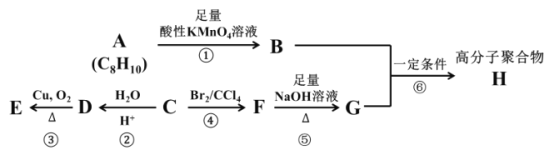
���У�ij�� C ����Ҫ��ʯ�ͻ�����Ʒ�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ�������� A ���ڷ��������ұ����ϵ�������ԭ�ӵ�Ч(������ͬ�Ļ�ѧ����)���߷��Ӿۺ��� H �ǹ�ҵ����Ҫ�ľ�����ά������������������Ȫˮƿ��������ά�������������Ϣ���ش��������⣺
(1)������ A �Ľṹ��ʽΪ _______��A �Ķ��ȴ�����____�֣�A �ķ�������������____��ԭ�ӹ�ƽ�档
(2)��Ӧ�ڵķ�Ӧ������ _____�������� D �й����ŵ�����Ϊ ______��
(3)��д����Ӧ�۵Ļ�ѧ��Ӧ����ʽ______��
(4)��д����Ӧ�Ļ�ѧ��Ӧ����ʽ______����ָ���÷�Ӧ�ķ�Ӧ���ͣ�_____��
(5)��ȡ�������廯���� I �ǻ����� B ��ͬ���칹�壬����������������
���ܷ���������Ӧ���ڲ�����NaHCO3 ��Һ��Ӧ����CO2���۱�����ֻ����һ��ȡ����
��д�� I ���������ֿ��ܵĽṹ��ʽ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��֪ˮ��25���100��ʱ�������ƽ��������ͼ��ʾ��

����25��ʱˮ�ĵ���ƽ������ӦΪ_____��(����A������B��)
��25���£�����������Һ�У���ˮ������������ӵ����ʵ���Ũ��֮��a:b:c=_____��
a��pH=1������b��pH=2������c��pH=12��NaOH��Һ
��25���£���VaLpH��a��������VbLpH��b��NaOH��Һǡ���кͣ�a+b=13����Va:Vb��_____��
(2)ij�¶�(t��)ʱ�����0.01mol��L��1��NaOH��Һ��pH��13��
�ٸ��¶���ˮ��Kw��_____��
�ڴ��¶��£���pH��1��������ҺVaL��pH��14��NaOH��ҺVbL���(��������仯)����Ϻ���Һ��pHΪ2����Va:Vb��_____��
(3)����˵������ȷ����_____��
a��25��ʱ��pH=12�İ�ˮ��pH=2��H2SO4��Һ�������ϣ�������ҺpH>7
b��100��ʱ��pH=12��NaOH��Һ��pH=2��H2SO4��Һǡ���кͣ�������ҺpH=7
c��25��ʱ����ˮ�������������Ũ��Ϊ1��10��10mol/L����Һ�п��ܴ�������NH4+��Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.1320mol/L��HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ��
ʵ���� | ����NaOH��Һ�����/mL | HCl��Һ�����/mL |
1 | 25.00 | 24.41 |
2 | 25.00 | 24.39 |
3 | 25.00 | 24.60 |
�ش��������⣺
��1����ͼ�м�Ϊ___________�ζ��ܣ���Ϊ_________ �ζ��ܣ����ʽ����ʽ ����
��2��ʵ���У���Ҫ��ϴ�������ǣ�________________________
��3��ȡ����ҺNaOH��Һ25.00ml ����ƿ�У�ʹ�÷�̪��ָʾ�����ζ��յ���ж�������________________________________________
��4�����ζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ����ʹ������____________���ƫ�ߡ���ƫ�͡������䡱����ͬ����������ʽ�ζ��ܶ���ʱ���ζ�ǰ���Ӷ������ζ�����ȷ��������������___________��
��5�� δ֪Ũ�ȵ�NaOH��Һ�����ʵ���Ũ��Ϊ_____________mol/l��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧʵ��С��Ϊ��̽�����������������������ʵ�鷽����
��֪�����������백����Ӧ2Al+2NH3![]() 2AlN+3H2��
2AlN+3H2��
�ڵ����������ȶ�����������ˮ���ᷴӦ���ڼ���ʱ����Ũ��ɲ���������
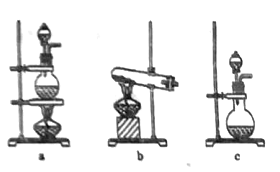
(1)��ͼ��ʵ�����Ʊ������ij��÷���װ�ã���ѡ��װ��b����÷���װ���еĻ�ѧ��Ӧ����ʽΪ��___________________________��
(2)����װ��c�Ľ�������ͼ��ʾ���Ӻ�װ�ã����װ�������ԣ���������ƿ�м�����ʯ�ң���Һ©���м���Ũ��ˮ��װ��G��ʢװ��ʯ�ң�װ��H�м������ۡ���װ��F����Һ©����������װ���п����ž����ٵ�ȼװ�DH���ƾ��ơ�
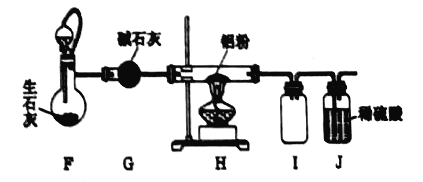
��װ��F��װ��c��ȵ��ŵ㣺 ____________________________��
����ƽ���ƶ�ԭ������װ��F�в���������ԭ�� __________________________��
��Ϊ�˵õ������ĵ��������ɽ�װ��H�й�����ȴ��ת�����ձ��У�����_______�ܽ⡢__________(���������)��ϴ�ӡ����T�ɡ�
�ܵ�����������������Һ���ȷ�Ӧ�����ӷ���ʽ�� __________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com