
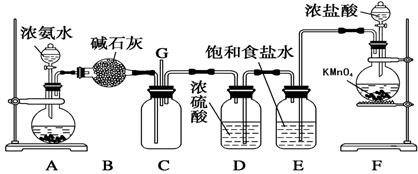
 NH3��H2O
NH3��H2O NH4����OH�������Ҫ�õ���������ƽ��������淴Ӧ������У�����A�еĹ�������Ǽ�ʯ�һ���ʯ�ҡ�
NH4����OH�������Ҫ�õ���������ƽ��������淴Ӧ������У�����A�еĹ�������Ǽ�ʯ�һ���ʯ�ҡ�

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ˮ��ŨH2SO4 | B������Na2CO3��Һ��ŨH2SO4 |
| C������NaCl��Һ��ŨH2SO4 | D������NaHCO3��Һ��ŨH2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 [����һ]
[����һ] ��ʵ�鷽��������þ�Ͻ�������NaOH��Һ��Ӧ���ⶨʣ�����������
��ʵ�鷽��������þ�Ͻ�������NaOH��Һ��Ӧ���ⶨʣ����������� ʵ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
ʵ���з�����Ӧ�Ļ�ѧ����ʽ�� �� ��ʵ�鲽�衽
��ʵ�鲽�衽 ��1����ȡ5.4g��þ�Ͻ��ĩ��Ʒ������V mL 2.0 mol/L NaOH��Һ�С�Ϊʹ�䷴Ӧ��ȫ����NaOH��Һ�����V �� ��
��1����ȡ5.4g��þ�Ͻ��ĩ��Ʒ������V mL 2.0 mol/L NaOH��Һ�С�Ϊʹ�䷴Ӧ��ȫ����NaOH��Һ�����V �� �� ��2�����ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���þ������������
��2�����ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���þ������������  (�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)�� [������
[������
 ]
]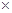 105Pa���������
105Pa��������� ���������ۡ���1��ͬѧ����ѡ������ʵ��װ�����ʵ�飺
���������ۡ���1��ͬѧ����ѡ������ʵ��װ�����ʵ�飺





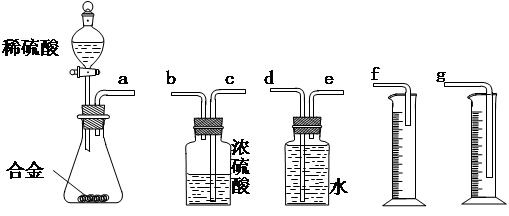

 ������Ϊ�����װ��������˳���ǣ�a�ӣ� ���� ���ӣ� ���� ���ӣ� ������ӿ���ĸ���ɲ���������
������Ϊ�����װ��������˳���ǣ�a�ӣ� ���� ���ӣ� ���� ���ӣ� ������ӿ���ĸ���ɲ��������� ��ʵ�鿪ʼʱ���ȴ�Һ©���ϿڵIJ��������������Һ©������ת�Ļ�����һ�����ϡ����Ҳ����˳��������ƿ�С������������ԭ�� ��
��ʵ�鿪ʼʱ���ȴ�Һ©���ϿڵIJ��������������Һ©������ת�Ļ�����һ�����ϡ����Ҳ����˳��������ƿ�С������������ԭ�� �� ��ʵ�����ʱ���ڶ�ȡ����ʵ�����������������ʱ������Ϊ�������˳���� ��
��ʵ�����ʱ���ڶ�ȡ����ʵ�����������������ʱ������Ϊ�������˳���� ��
A���ȴ�ʵ��װ����ȴ | B�������ƶ���Ͳf��ʹ����Һ������ƿ��Һ����ƽ | C�������ƶ���Ͳg��ʹ����Һ������ƿ��Һ����ƽ | D�������밼Һ�����͵�ˮƽ��ȡ��Ͳ��ˮ����� |
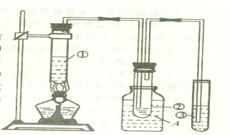
 ��2����ϸ����ʵ��װ�ú�ͬѧ�Ǿ�������Ϊ�������������ϴ���ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС�����������������ͼ��ʾ��ʵ��װ�á�
��2����ϸ����ʵ��װ�ú�ͬѧ�Ǿ�������Ϊ�������������ϴ���ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС�����������������ͼ��ʾ��ʵ��װ�á�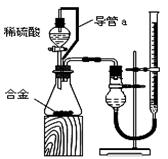
 ��װ���е���a�������� ��
��װ���е���a�������� �� ��ʵ��ǰ���ʽ�ζ�����Һ������ֱ�ΪV1 mL��V2 mL����������������Ϊ_________mL��
��ʵ��ǰ���ʽ�ζ�����Һ������ֱ�ΪV1 mL��V2 mL����������������Ϊ_________mL��



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
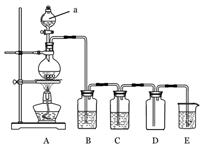
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

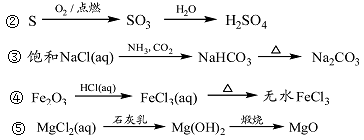
| A���٢ۢ� | B���ڢۢ� | C���ڢܢ� | D���٢ܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com