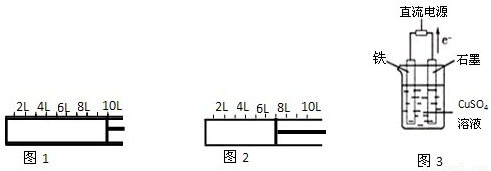
I����ͼ1��ʾ����2molA�����1molB�������һ�ݻ��ɱ���ܱ������У�������Ӧ��2A��g��+B��g��?2C��g������Ӧ��ʼʱ�ɻ����Ļ�����λ����ͼ1��ʾ������Ӧ�ﵽƽ��ʱ������λ����ͼ2��ʾ����ﵽƽ��ʱ��A��ת����Ϊ______���������·�Ӧ��ƽ�ⳣ��Ϊ______��
��1����֪298Kʱ��1molC
2H
6����������ȫȼ�����ɶ�����̼��Һ̬ˮ���ų�����1558.3KJ��д���÷�Ӧ�Ļ�ѧ����ʽ______��
��2�����ø÷�Ӧ���һ��ȼ�ϵ�أ�������������Һ����� ����Һ���ö��ʯī���缫���ڵ缫�Ϸֱ���������������д�������ĵ缫��Ӧʽ______��
��3���л�ѧʵ��װ����ͼ3��ʯī���ϵĵ缫��ӦʽΪ______�������ʼʱʢ��1000mLpH=5������ͭ��Һ��25�棩��CuSO
4 ��������һ��ʱ�����Һ��pH��Ϊ1����Ҫʹ��Һ�ָ�����ʼŨ�ȣ�������Һ����ı仯����������Һ�м���______�����������ƣ���������Ϊ______��




 ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
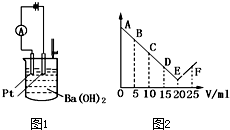 ��ͼ1��ʾ����30ml 0.1mol?L-1Ba��OH��2��Һ�����ձ��У�Ȼ����������0.1mol?L-1ij��������Ԫ���γɵ�ij����Һ��25mL����������Һ�����V�����ǿ��I��I-Vͼ��ͼ2��ʾ��
��ͼ1��ʾ����30ml 0.1mol?L-1Ba��OH��2��Һ�����ձ��У�Ȼ����������0.1mol?L-1ij��������Ԫ���γɵ�ij����Һ��25mL����������Һ�����V�����ǿ��I��I-Vͼ��ͼ2��ʾ��