
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50mL 0.25mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL 0.55mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
����NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ������¶ȡ�
�ش��������⣺
��1������A������Ϊ_________________________��
��2������NaOH��Һ����ȷ������________��
A���ز����������� B���������������� C��һ��Ѹ�ٵ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������________��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β���������ؽ���
��4��ʵ���������±���
������д�±��еĿհף�
�¶� ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ �¶� t2/�� | �¶Ȳ� ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | _______ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�����Ƶ���Ϊ0.55mol/L NaOH��Һ��0.25mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к�����H �� ______ (ȡС�����һλ)��
���к��Ȳⶨʵ���У����в���һ���ή��ʵ��ȷ�Ե���________��
A���õζ���(��������������������0.01)ȡ���������Һ�����
B��NaOH��Һ�ڵ���С�ձ�ʱ������������
C����С�ձ�������ϴв��ŵ�����ĭ���Ͻ϶�
D������HCl��Һ���¶ȼ���ˮϴ�����������NaOH��Һ���¶�
���𰸡����β�������� C D 3.4 ��56.8kJ/mol B
��������
��1������װ��ͼ��������A��������(2)��NaOH��Һ����С�ձ��У��ּ��ε��룬�ᵼ������ɢʧ��Ӱ��ⶨ�����(3)������������ƻ��ʱ���������¶ȼ��ϵĻ��β������������ؽ�����ʹ������NaOH��Һ��Ͼ����� (4) �ٵ�һ��ʵ����¶Ȳ���3.4���ڶ���ʵ����¶Ȳ���5.1��������ʵ����¶Ȳ���3.3�����Ĵ�ʵ����¶Ȳ���3.5�����ڵڶ���ʵ������ƫ������ֵ��ֻ�ܸ��ݵ�1��3��4��ʵ������¶Ȳ��ƽ��ֵ���ڸ��ݼ��㹫ʽQ=cm��T����ʵ��ų����������к�����ǿ��ǿ��ϡ��Һ��ȫ��Ӧ����1molˮ�ų�����������NaOH��Һ�ڵ���С�ձ�ʱ��������������ʵ��������ˮ�������٣��ų���������С������к��ȵ���ֵ����
��1������װ��ͼ������A�������ǻ��β����������(2)��������������Һʱ������һ��Ѹ�ٵĵ��룬Ŀ���Ǽ���������ɢʧ�����ּܷ��ε�������������Һ������ᵼ������ɢʧ��Ӱ��ⶨ�������ѡC�� (3)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ����������:�������¶ȼ��ϵĻ��β������������ؽ������¶ȼ��Dz����¶ȵģ�����ʹ���¶ȼƽ�����Ҳ������������ձ���������ܵ���Һ�彦��������ɢʧ��Ӱ��ⶨ����������ܴ�ӲֽƬ�ò��������裬�����������ɢʧ����ѡD��(4) �ٵ�һ��ʵ����¶Ȳ���3.4���ڶ���ʵ����¶Ȳ���5.1��������ʵ����¶Ȳ���3.3�����Ĵ�ʵ����¶Ȳ���3.5�����ڵڶ���ʵ������ƫ������ֵ��ֻ�ܸ��ݵ�1��3��4��ʵ������¶Ȳ��ƽ��ֵ��![]() ����50mL��0.50mol/L������50mL��0.55mol/L����������Һ��������Ϊm=100mL��1g/cm3=100g��c=4.18 J/(g����)�����빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18 J/(g����)��100g��3.4��=1.42kJ��������0.025mol��ˮ�ų�����Ϊ1.42kJ����������1mol��ˮ�ų�����Ϊ1.42kJ��
����50mL��0.50mol/L������50mL��0.55mol/L����������Һ��������Ϊm=100mL��1g/cm3=100g��c=4.18 J/(g����)�����빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18 J/(g����)��100g��3.4��=1.42kJ��������0.025mol��ˮ�ų�����Ϊ1.42kJ����������1mol��ˮ�ų�����Ϊ1.42kJ��![]() =56.8kJ������ʵ���õ��к��ȡ�H=-56.8kJ/mol����A���õζ��ܣ���������������������0.01��ȡ���������Һ��������������ȷ�����ʵ��ȷ�ԣ� B������������Һ����ʱ����������٣���ʹ���к�������ƫС������һ���ή��ʵ��ȷ�ԣ� C����С�ձ��в��ŵ�����ĭ���Ͻ϶࣬����Ч�����ã������ʵ��ȷ�ԣ� D������HCl��Һ���¶ȼ���ˮϴ���ٲ��������ƣ��������ͼ�֮����Ϊ�кͷ�Ӧ�����µ�������ʧ�����ʵ���ȷ��������������B�������⣬ѡB.
=56.8kJ������ʵ���õ��к��ȡ�H=-56.8kJ/mol����A���õζ��ܣ���������������������0.01��ȡ���������Һ��������������ȷ�����ʵ��ȷ�ԣ� B������������Һ����ʱ����������٣���ʹ���к�������ƫС������һ���ή��ʵ��ȷ�ԣ� C����С�ձ��в��ŵ�����ĭ���Ͻ϶࣬����Ч�����ã������ʵ��ȷ�ԣ� D������HCl��Һ���¶ȼ���ˮϴ���ٲ��������ƣ��������ͼ�֮����Ϊ�кͷ�Ӧ�����µ�������ʧ�����ʵ���ȷ��������������B�������⣬ѡB.



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ʼ�ķ�Ӧ���ܹ�˵������Ӧ���������¶ȡ�Ũ�ȡ������ȣ���ͬ�õ����ﲻͬ���Ĺ۵���ǣ� ��
A.��������ˮB.����������
C.����������D.������̼�ͳ���ʯ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й���֬����������ȷ���ǣ� ��
A.��Ȼ��֬�ǻ���û�й̶����۵�ͷе�
B.��֬������һ��
C.��֬���ɸ�֬������������ɵ���
D.��֬������ʹ��ˮ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.1000mol/L��NaOH��Һ�ֱ�μӵ�20.00mLŨ�Ⱦ�Ϊ0.1000mol/L��һԪ��HX��HY��Һ�У���Һ��pH������NaOH����Ĺ�ϵ��ͼ��ʾ�����������������
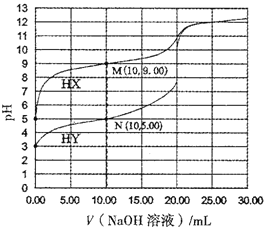
A. N����Һ��c(Y��)>c(Na+)>c(OH��)>c(H+)
B. �����£�0.1000mol/LHY��Һ�ĵ����ԼΪ1%
C. Ka(HX)������Ϊ10��9
D. ��NaOH��Һ�ζ�HY���÷�̪��ָʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��N2(g) + 3H2(g)![]() 2NH3(g) ��H = 92 kJ��mol-1����ͼ��ʾLһ��ʱ��H2��ƽ��ת����(��)��X�ı仯��ϵ��L(L1��L2)��X�ɷֱ����ѹǿ���¶ȡ�����˵���У�����ȷ����
2NH3(g) ��H = 92 kJ��mol-1����ͼ��ʾLһ��ʱ��H2��ƽ��ת����(��)��X�ı仯��ϵ��L(L1��L2)��X�ɷֱ����ѹǿ���¶ȡ�����˵���У�����ȷ����

A��X��ʾ�¶�
B��L2��L1
C����Ӧ���� ��(M)����(N)
D��ƽ�ⳣ�� K(M)��K(N)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ͼʾװ���Ʊ�KC1O��Һ������KOH��Fe(NO3)3��Һ��Ӧ�Ʊ���Ч��ˮ��K2FeO4��
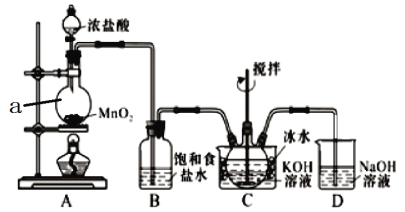
���������ϣ��� Cl2��KOH��Һ��20�����·�Ӧ����KClO���ڽϸ��¶���������KClO3���� K2FeO4������ˮ������ŨKOH��Һ����0����5���ǿ������Һ�н��ȶ���
(1)����a��������_________��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ__________________��
(2)װ��C��������ƿ���ڱ�ˮԡ�е�Ŀ����_________________________________��
(3)װ��B���յ�������_________��װ��D��������___________________________��
(4)C�еõ�����KC1O������ƿ�ϵĵ���ȡ�£����μ���KOH��Һ��Fe(NO3)3��Һ��ˮԡ���Ʒ�Ӧ�¶�Ϊ25�棬����1.5h����Һ��Ϊ�Ϻ�ɫ(��K2FeO4)���÷�Ӧ�����ӷ���ʽΪ__________________________��
(5)��(4)������Һ�м��뱥��KOH��Һ����ȴ��0����5�������Ϻ�ɫ���壬���ˣ��õ�K2FeO4�ֲ�Ʒ��K2FeO4�ֲ�Ʒ����KCl�����ʣ���һ���ᴿ������___________��
(6)��ʵ����������VmL c mol/L��Fe(NO3)3��Һ��Fe(NO3)3��ַ�Ӧ�������Ƶ�a g��K2FeO4���壬��ʵ��K2FeO4�IJ���Ϊ ______________(�г���ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���һ�ֿ�������Դ����CO2�Ʊ��״��Ĺ��̿����漰�ķ�Ӧ���£�
��Ӧ����CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1=��49.58 kJmol��1
CH3OH(g)+H2O(g) ��H1=��49.58 kJmol��1
��Ӧ����CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2
CO(g)+H2O(g) ��H2
��Ӧ����CO(g)+2H2(g)![]() CH3OH(g) ��H3=��90.77 kJmol��1
CH3OH(g) ��H3=��90.77 kJmol��1
�ش��������⣺
(1)��Ӧ��ġ�H2=_________������Ӧ����������ƽ�ⳣ���ֱ�ΪK1��K2��K3����K2=________(��K1��K3��ʾ)��
(2)��Ӧ���Է�����������___________(�����ϵ��¶��������ϸ��¶��������κ��¶���)��
(3)��һ��������2 L�����ܱ������г���3 mol H2��1.5 mol CO2����������Ӧ��ʵ���ò�ͬ��Ӧ�¶�����ϵ��CO2��ƽ��ת���ʵĹ�ϵ�����±���ʾ��
�¶�(��) | 500 | T |
CO2��ƽ��ת���� | 60�G | 40�G |
��T______500��(����>������<�� ����=��)��
���¶�Ϊ500��ʱ���÷�Ӧ10 minʱ�ﵽƽ�⡣��H2��ʾ�÷�Ӧ�ķ�Ӧ����v(H2)=______________�����¶��£���ӦI��ƽ�ⳣ��K=______________L2/mol2
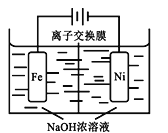
(4)��CO2�Ʊ��״�����Ҫ��������ҵ���õ�ⷨ��ȡNa2FeO4��ͬʱ���������Fe+2H2O+2OH![]() FeO42+3H2��������ԭ����ͼ��ʾ�����һ��ʱ���c(OH)���͵�������__________ (������������������������)�����ҷ����ĵ缫��ӦʽΪ��___________________________��
FeO42+3H2��������ԭ����ͼ��ʾ�����һ��ʱ���c(OH)���͵�������__________ (������������������������)�����ҷ����ĵ缫��ӦʽΪ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã�����ѧ�����ش��������⡣

��1��Ԫ�آٵ�ԭ�ӽṹʾ��ͼΪ_________��
��2��Ԫ�آۺ͢��γɻ�����õ���ʽ��ʾ�γ������_________��
��3��Ԫ�آڡ����γɼ����ӵİ뾶�������ӷ��ţ�_________>_________��
��4��Ԫ�آڡ����γɵ���̬�⻯���ȶ��ԣ��ѧʽ����ͬ��______>_______��Ԫ�آۡ����γɵ����������ˮ����ļ���_________>_________��
��5��Ԫ�آܵ������������Ԫ�آ�����������ˮ����ϡ��Һ��Ӧ�����ӷ���ʽ__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������ķ��Ӹ���Ϊ1.204��1023�����������ʵ���Ϊ_____mol��������ԭ�Ӹ�������______��������Щ����������ȵ�O2����ԭ�ӵ����ʵ���Ϊ______��
��2�����³�ѹ�£�2.8gCO��N2��������к��е�ԭ������Ϊ_____������ԭ��������Ϊ0.8g����CO��N2�������Ϊ_____��
��3��ͬ��ͬѹ�£�������ͬ��ԭ����Ŀ��CO��CO2���������ܶ�֮��Ϊ_____������֮��Ϊ______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com