
�����Ǽ�����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͻ�������NO2���ܱ���������ˮ��η���ѭ�������Ʊ����ᡣ
��1����ҵ����ˮ����NO2����HNO3�����ɵ����徭������������յ�ѭ���������ת��Ϊ���ᣨ�ٶ�����������������ʧ������д��������Ӧ�Ļ�ѧ����ʽ��___________________________________________��
��2��Ϊ��֤��NOҲ������������ˮ��ͬ��Ӧ����HNO3��ijѧ���������ͼ��ʾװ�ã��йؼг�װ������ȥ����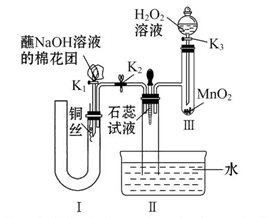
�ټ��װ�����������ú�Ϊ�۲쵽NO�������ɣ���K1���ر�K2��Ӧ��U�ιܵij��ܿ�ע��ϡ������____________��Ѹ�ٹر�K1���۲쵽U�ι��ڵ�������_______________________________________________________________��
��װ�â��з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��պNaOH��Һ�����ŵ�������_______________________________________��
�ܴ�K2����װ�â��г��������е��������ɫ��K3����Ӧһ��ʱ����������в�δ����Һ�塣��Ƽ������鳤�������е������Ƿ�NO________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ƕ���Ҫ�ǽ������仯��������ۣ�����Ҫ��ش����⣺
��1��ʵ����ʢװNaOH��Һ���Լ�ƿ�����ò�������Ӧ�����������Է�ֹ������Ӧ��
�����ӷ���ʽ����
��2������һ����Ҫ�Ļ�����Ʒ���ܶȱȿ��� �����С��������ҵ���Ʊ������Ļ�ѧ����ʽΪ ��
��3����ҵ����ȡƯ�۵ķ�Ӧ��ѧ����ʽΪ ��
��4��ŨH2SO4��������������������Ϊ������____________�������������Ƿ����ձ��У����뼸��ˮ��������ȡ�Ȼ���������Ũ���ᣬѸ�ٽ��裬�ų��������ȣ�ͬʱ�۲쵽������ڣ�������ͣ����ų��д̼�����ζ�����塣��ش�
�����̼�����ζ����Ļ�ѧ����ʽΪ ��
��5��ͭ��ϡ���ᷴӦ�����ӷ���ʽ�� �����μӷ�Ӧ��Cu����Ϊ6.4g������NO����____________L����״���£�����ת�Ƶ������ʵ���Ϊ mol������ԭ����δ����ԭ��HNO3���ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᡣ������ͼת����ϵ(��Ӧ���������ֲ�������ȥ)���ش��������⣺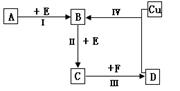
(1)��A��B��C��D��Ϊ��XԪ�صĻ������A��F��һ�������ж�ֻ����10�����ӣ���
��A���ӵĿռ乹��Ϊ ��F���ӵĵ���ʽΪ ��
�ڷ�Ӧ I �Ļ�ѧ����ʽΪ ��
�ۻ�����NaX3�Ǻϳɡ���ơ����м�������ʣ�Ҳ��������ȫ�����е���Ҫ���ʡ�NaX3��ײ��������Na3X����һ�����嵥�ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
�� X�����������γɹ⻯ѧ����ԭ��֮һ����ҵ�Ͽɲ����������⻯�ﷴӦ�����������ʶ���ȥ�����÷���ʽ��ʾ�÷�Ӧ ��
(2)��A��B��C��D��Ϊ��YԪ�صĻ��������A������Ԫ����ɣ���A��Ħ������Ϊ120g��mol�C1����
�ٽ���ӦIV���õ���Һ�������ɵõ��ľ������� ����(����ӡ��������ӡ�����ԭ�ӡ�)
�ڷ�Ӧ I �Ļ�ѧ����ʽΪ ��
�ۺ�YԪ�صĻ�����Na2Y�ʹ���������Һ��ǿ���Ի������ܷ�����Ӧ��������������д���÷�Ӧ�����ӷ�Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(20��)ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��Cl2��ijЩ���ʣ���ͬѧ�������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⣺
��1�����з����У����Ƶõ���ȷ�����__________��
��MnO2��Ũ�����Ϲ��ȣ� ��MnO2��NaCl��Ũ�����Ϲ��ȣ�
��NaClO��Ũ�����ϣ� ��K2Cr2O7��Ũ�����ϣ�
��KClO3��Ũ�����Ϲ��ȣ� ��KMnO4��Ũ�����ϡ�
A���٢ڢ�B���ڢܢ�C���٢ܢ� D��ȫ������
��2��д��ʵ������ȡCl2�����ӷ���ʽ____________��
��3�����ú���0.2 mol HCl��Ũ������������MnO2��Ӧ�Ƶ�Cl2�����������£�����С��1.12L��ԭ����_________________________________________��
��4����װ��B��������__________________________________��
��װ��C��D���ֵIJ�ͬ����˵����������________________________��
��װ��E��������_____________________��
��5����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����ȷ������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֣���ͬѧ���Ӧ����װ��__________��________֮�䣨��װ����ĸ��ţ�����һ��װ�ã�����װ��������Լ���Ϊ____________��
A��ʪ��ĵ���KI��ֽB������������Һ
C��ʪ��ĺ�ɫ���� D�����͵�ʳ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��1����A����������ˮ������D�����������������������;���Ľ������ʣ���������B����Һ���ܵõ�B����B�Ļ�ѧʽ������ ����ҵ����ȡA�����ӷ���ʽ��Ϊ ��
��2����A��һ�ּ������壬�������������B������β��֮һ�����������ɫ����Ӧ�ٵĻ�ѧ����ʽΪ ��
��3����D���ȼҵ����Ҫ��Ʒ��B�����ԣ���Ӧ�ڵ����ӷ���ʽ�� ��
��4����A��C��D���dz������壬C���γ��������Ҫ���壬��Ӧ�۵Ļ�ѧ����ʽ ��
��5���ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խڣ��磩��30%���ϣ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ����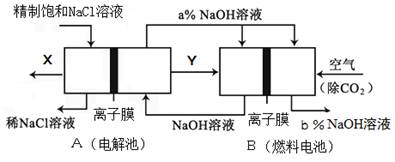
��6�����ͼ��X��Y�ֱ��� ���ѧʽ���������Ƚ�ͼʾ������������������a% b%�����������=��������
��д��ȼ�ϵ��B�и����Ϸ����ĵ缫��Ӧ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��19��2 g��Cu����������ϡ�����У�����Cu��ȫ��Ӧ��
��֪��3Cu + 8HNO3(ϡ) = 3Cu(NO3)2 +2NO��+ 4H2O��
��1���μӷ�Ӧ����������ʵ�����
��2������ԭ�������������
��3�����ɵ�NO�ڱ�״���µ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
������һ����Ҫ�Ĺ�ҵԭ�ϣ���ҵ������Ĺؼ��ǰ��Ĵ������������Ṥҵ��صĹ����в����ĵ�������Ĵ�����Ӧ��Ҳ�ǿ�ѧ�о����ȵ㡣
I��ͼ10��ͼ11�ֱ���ʵ����ģ��ϳɰ�������������װ��
��1������������ͨ��ͼ10װ�ã���װ����Ũ����������ǿ����������ٺ� ��
��2����ͼ11װ������һ��ʱ�䰱����ͨ�������ͬʱ���Ѿ����ȵIJ�˿������װ�õ���ƿ�ڣ���˿���ֺ��ȵ�ԭ���� ��д����װ���а������Ļ�ѧ����ʽ ����Ӧ��������ƿ�ڵ���Һ�к���H����OH���� ���ӡ� ���ӡ�
II�������й�������ʵ�Ľ��ͺ�������
| A��Ũ����ͨ����������ɫ���Լ�ƿ�У�˵��Ũ����ȶ� |
| B����������ϡ���ᷴӦ����Һ��dz��ɫ��˵��ϡ����������������� |
| C������Ũ������ͭм��Ӧ����ȡ����ͭ��˵��Ũ������лӷ��� |
| D������п��ϡ���ᷴӦ��ȡ������˵��ϡ�����ܽ�п�ۻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ�о�ͭ��Ũ����ķ�Ӧ��ij��ѧ��ȤС���������ʵ�顣
ʵ���Ӧ����Ķ���̽����
ʵ��װ����ͼ��ʾ�����̶�װ������ȥ��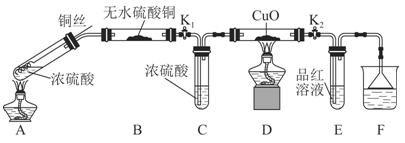
��1��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��2��F�ձ��е���Һͨ���� ��
��3��ʵ������У���֤��Ũ��������Ԫ�ص�������ǿ����Ԫ�ص�������
��
��4��ʵ�������֤��Aװ���Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ��������� ��
��5��Ϊ˵��Ũ�����е�ˮ�Ƿ�Ӱ��Bװ��������жϣ��������һ��ʵ�顣ʵ�鷽��Ϊ ��
ʵ���Ӧ����Ķ���̽��
��6����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣�������������������ϡ�
����1��
| ����/mol��L��1 | ��ɫ���ʳ��ֵ��¶�/�� | ��ɫ������ʧ���¶�/�� |
| 15 | Լ150 | Լ236 |
| 16 | Լ140 | Լ250 |
| 18 | Լ120 | ����ʧ |
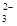 ��I2===S4O
��I2===S4O ��2I����
��2I�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��̽��AgNO3�����ȶ��ԣ�ij��ѧ��ȤС�����������ʵ�顣����ͼ��ʾ��ʵ��װ��A����AgNO3���壬��������ɫ���壬��װ��D���ռ�����ɫ���塣����Ӧ�������Թ��в�������Ϊ��ɫ��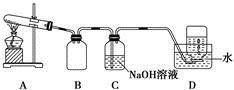
(1)װ��B�������� ��
(2)��С�����۲���֤����ɫ����ΪO2������֤������ ��
(3)���������ϡ�Ag2O�ͷ�ĩ״��Ag��Ϊ��ɫ��Ag2O�����ڰ�ˮ��
��������롿�Թ��в����ĺ�ɫ��������ǣ���.Ag����.Ag2O����.Ag��Ag2O��
��ʵ����֤����С��Ϊ��֤�������룬�ֱ�ȡ������ɫ��������Թ��У�����������ʵ�顣
| ʵ���� | ���� | ���� |
| a | ����������ˮ���� | ��ɫ���岻�ܽ� |
| b | ��������ϡ���ᣬ�� | ��ɫ�����ܽ⣬����������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com