
Ϊ��̽��AgNO3�����ȶ��ԣ�ij��ѧ��ȤС�����������ʵ�顣����ͼ��ʾ��ʵ��װ��A����AgNO3���壬��������ɫ���壬��װ��D���ռ�����ɫ���塣����Ӧ�������Թ��в�������Ϊ��ɫ��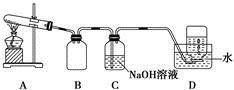
(1)װ��B�������� ��
(2)��С�����۲���֤����ɫ����ΪO2������֤������ ��
(3)���������ϡ�Ag2O�ͷ�ĩ״��Ag��Ϊ��ɫ��Ag2O�����ڰ�ˮ��
��������롿�Թ��в����ĺ�ɫ��������ǣ���.Ag����.Ag2O����.Ag��Ag2O��
��ʵ����֤����С��Ϊ��֤�������룬�ֱ�ȡ������ɫ��������Թ��У�����������ʵ�顣
| ʵ���� | ���� | ���� |
| a | ����������ˮ���� | ��ɫ���岻�ܽ� |
| b | ��������ϡ���ᣬ�� | ��ɫ�����ܽ⣬����������� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�����Ǽ�����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͻ�������NO2���ܱ���������ˮ��η���ѭ�������Ʊ����ᡣ
��1����ҵ����ˮ����NO2����HNO3�����ɵ����徭������������յ�ѭ���������ת��Ϊ���ᣨ�ٶ�����������������ʧ������д��������Ӧ�Ļ�ѧ����ʽ��___________________________________________��
��2��Ϊ��֤��NOҲ������������ˮ��ͬ��Ӧ����HNO3��ijѧ���������ͼ��ʾװ�ã��йؼг�װ������ȥ����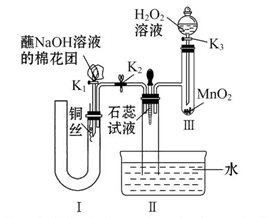
�ټ��װ�����������ú�Ϊ�۲쵽NO�������ɣ���K1���ر�K2��Ӧ��U�ιܵij��ܿ�ע��ϡ������____________��Ѹ�ٹر�K1���۲쵽U�ι��ڵ�������_______________________________________________________________��
��װ�â��з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��պNaOH��Һ�����ŵ�������_______________________________________��
�ܴ�K2����װ�â��г��������е��������ɫ��K3����Ӧһ��ʱ����������в�δ����Һ�塣��Ƽ������鳤�������е������Ƿ�NO________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(12��)ij�о�С����̽��SO2�Ļ�ѧ���ʣ����������ʵ�鷽����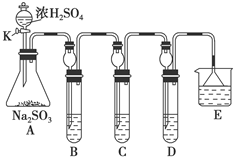
��1����B�м���SO2�������ԣ���B����ʢ�Լ���Ϊ________��
��2����C��װFeCl3��Һ������SO2�Ļ�ԭ�ԣ���C�з�Ӧ�����ӷ���ʽΪ__________________________��
��3����D��װ����Ư��Ũ��Һ��ͨ��SO2һ��ʱ���D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷֽ�����̽������ش��������⣺
��ѡ���������Լ�������װ�á��Թܡ��ιܡ������ܵĵ�����������ˮ��0��5 mol��L��1���ᡢ0��5 mol��L��1H2SO4��Һ��0��5 mol��L��1BaCl2��Һ��Ʒ����Һ�����Ƴ���ʯ��ˮ��
(��)����һ���ð�ɫ����ΪCaSO3��
��������ð�ɫ����Ϊ________��
���������ð�ɫ����Ϊ�����������ʵĻ���
(��)���ڼ���һ����д�±���
| ʵ����� | Ԥ������ͽ��� |
| ��D�г������ˣ�ϴ�Ӹɾ����� | |
| ����һ�ɾ��Թ�ȡ����������Ʒ������ ______ | __________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ȤС����Ƴ���ͼ��ʾװ�����Ľ��̲��С�ͭ�����ᷴӦ��ʵ�飬��̽����ѧʵ�����ɫ����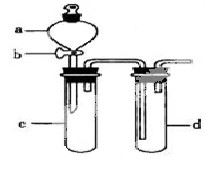
��1��ʵ��ǰ���رջ���b���Թ�d�м�ˮ����û�����ܿڣ������Թ�c��d�Ľ���������c����Ŀ����__________��
��2����d�м�����NaOH��Һ��c�з�һС��ͭƬ���ɷ�Һ©��a��c�м���2mLŨ���ᣬc�з�Ӧ�Ļ�ѧ����ʽ��______________________��
����a��c�м�2mL����ˮ��c�е�ʵ��������_____________��
��3���±�����ȡ����ͭ�����ַ�������������ɫ��ѧ�������ѷ�����_____��������_______��
| ���� | ��Ӧ�� |
| �� | Cu��Ũ���� |
| �� | Cu��ϡ���� |
| �� | Cu��O2��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�
��1�������̢��еIJ���������ͨ��������Һ�У���Һ����ɫ���� ��
A��Ʒ����Һ B����ɫʯ����Һ C������KMnO4��Һ D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ�� ��
��3�����̢��У������������� ��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ�������� ��
��5�����̢ݵ���pH��ѡ�������Լ��е� (��ѡ�����)��
A��ϡ���� B��CaCO3 C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70һ80�棬Ŀ���� ��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.700g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[(Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ ��(���������в�����Ԫ�غ���Ԫ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʡ��2013��12��1����ʱ�𣬳�����������Ϊ�������������Զ���������ŷ����˴��ĸ��ơ���֪SO2������Fe( NO3)3��Һ���գ� 0��1mol/L��Fe(NO3)3��Һ��pH��2��ijѧϰС��ݴ�չ���������̽����
��̽��I��ͭ��Ũ����ķ�Ӧ̽����
(l)ȡ12��8gͭƬ��������ƿ�У�ͨN2һ��ʱ����ټ���20 mL 18 mol?L-1��Ũ���ᣬ���ȡ�װ��A���а������������������ɣ�װ��B�в�����ɫ��������ַ�Ӧ��,��ƿ������ͭƬʣ�ࡣ
�ٸ�С��ͬѧ��Ϊ��ƿ�г���ͭƬʣ���Ӧ�н϶������ʣ�࣬��ԭ���ǣ� ___________________ ��
�ڸ�С��ͬѧ��ͨ���ⶨ�����������������������ʵ���������˶���ʵ�鷽�������з��������е���______ ��
A�������������建��ͨ��Ԥ�ȳ�����ʢ�м�ʯ�ҵĸ����,������Ӧ���ٴγ���
B�������������建��ͨ�����������ữ�ĸ��������Һ���ټ���������BaCl2��Һ���������ó���������
C�����ű���NaHSO3��Һ�ķ����ⶨ�������������(����ɱ�״����
��̽��II��װ��B�в���������ԭ��̽����
��2������Ũ����֮ǰ��ͨN2һ��ʱ�䣬��Ŀ����____ ��
��3���������ۣ���С���װ��B�в���������ԭ��������в���(�����Ǹ����صĵ��ӣ���
����1: װ��A�еİ�������B���뷴Ӧ
����2��SO2��Fe3+����ΪSO42-
����3�� ��
��4����ͬѧ��ΪֻҪ��װ��A��B������ϴ��ƿC���Ϳ����ų�װ��A�а���Ӱ�죬��C��ʢ�ŵ��Լ��� ��
��5����ͬѧȡ������װ��B����Һ�����뼸�����Ը�����أ������Ϻ�ɫ��ȥ���ݴ���Ϊ����2���������Ƿ�ͬ������ۣ���˵�����ɣ� ��
��˼���뽻����
��6��ʵ���������ʹ��ƿ��ͭƬ�����ܽ⣬���з���(��Ҫʱ�ɼ��ȣ����е��� ��
A�����ɼУ�ͨ��O2 B���ɷ�Һ©������H2O2��Һ
C���ɷ�Һ©������NaNO3��Һ D���ɷ�Һ©������Na2SO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��Ϊ��֤NO2�������Ժ�NO�Ļ�ԭ�ԣ����������װ����ȡNO2��NO������֤�����ʣ�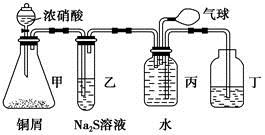
(1)д�����з�Ӧ�����ӷ���ʽ��_____________________
���������__________________________________________________
��֤��NO2�������ԣ��ڱ��й���������������_____________________________________����֤��NO�Ļ�ԭ�ԡ�
(2)ʵ��ǰ���г���ˮ��������________________________________________
(�÷�Ӧ�Ļ�ѧ����ʽ�ͼ�Ҫ���ֻش�)��
(3)С��������ʵ�������������ɣ�����Ϊ���е���������֤��NO2�������ԣ�����������_________________________________________________��
����Ϊ��������ȷ֤��NO2�������ԣ�___________________________
(��Ҫ�ش��ԭ��������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������Ũ�����ϼ��ȵõ���������ͼ����ȡ��̽��Cl2��ѧ���ʵ�װ��ͼ��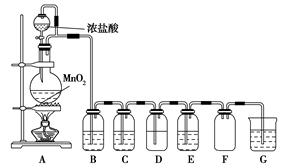
(1)Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2)��Ҫ�õ����﴿�������壬B��C��Ӧ�ֱ�ʢ�ŵ��Լ�Ϊ �� ��
(3)E����װ��FeCl2��Һ����Ӧ�����ӷ���ʽΪ ��E����װ�е��۵⻯����Һ���ܹ۲쵽��ʵ�������� ��
(4)ʵ���з��֣�Ũ������MnO2��ϼ�������������ϡ������MnO2��ϼ��Ȳ����������������������ij��ѧ��ȤС��ԡ�Ӱ���������ɵ�ԭ���������ۣ������������ʵ�鷽����
a��ϡ�������MnO2�У�Ȼ��ͨ��HCl�������
b��ϡ�������MnO2�У�Ȼ�����NaCl�������
c��ϡ�������MnO2�У�Ȼ�����Ũ�������
d��MnO2��NaCl��Ũ��Һ��ϼ���
e��Ũ������NaCl���塢MnO2���干��
��ʵ��b��Ŀ���� ��ʵ��c��Ŀ���� ��
��ʵ������a��c��e�л���ɫ�������ɣ�b��dû�л���ɫ�������ɡ��ɴ˵ó�Ӱ���������ɵ�ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ����С��ͨ��ʵ���о�NO2�����ʡ�
��֪:2NO2+2NaOH NaNO3+NaNO2+H2O
NaNO3+NaNO2+H2O
����1:������ͼ��ʾװ��̽��NO2�ܷ�NH3��ԭ(K1��K2Ϊֹˮ��,�г̶ֹ�װ����ȥ)��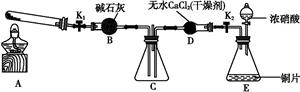
(1)Eװ������ȡNO2��Ӧ�Ļ�ѧ����ʽ�� ����
(2)��NO2�ܹ���NH3��ԭ,Ԥ�ڹ۲쵽Cװ���е��������� ��
(3)ʵ�������,δ�ܹ۲쵽Cװ���е�Ԥ������С��ͬѧ�ӷ�Ӧԭ���ĽǶȷ�����ԭ��,��Ϊ������:
��NH3��ԭ�Խ���,���ܽ�NO2��ԭ;
���ڴ�������,NO2��ת���ʼ���;
���� ��
(4)��ʵ��װ�ô���һ�����Ե�ȱ���� ��
����2:̽��NO2�ܷ���Na2O2����������ԭ��Ӧ��
(5)ʵ��ǰ,��С��ͬѧ������ּ��衣
����1:���߲���Ӧ;
����2:NO2�ܱ�Na2O2����;
����3:���������������������� ��
(6)Ϊ����֤����2,��С��ͬѧѡ������1�е�B��D��Eװ��,��B�е�ҩƷ����ΪNa2O2,��ѡFװ��(��ͼ��ʾ),������װ,����ʵ�顣
��װ�õĺ�������˳������ ��
��ʵ�������,Bװ���е���ɫ��ĩ��ɰ�ɫ��������,�ð�ɫ����Ϊ������,���������������ɡ��Ʋ�Bװ���з�Ӧ�Ļ�ѧ����ʽΪ����������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com