
�����й�NaClO��NaCl�����Һ��������ȷ���ǣ� ��
A������Һ�У�H+��NH4+��SO42-��Br-���Դ�������
B������Һ�У�Ag+��K+��NO3-��CH3CHO���Դ�������
C�������Һ�е�������FeSO4��Һ����Ӧ�����ӷ���ʽΪ��2Fe2++ClO-+2H+ = Cl-+2Fe3++H2O
D�������Һ�м���Ũ���ᣬÿ����1molCl2��ת�Ƶ���ԼΪ6.02��1023��
 һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�߶���12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���о����������ȷ��һ���� ( )��
ѡ�� | ���Ӿ��� | ԭ�Ӿ��� | ���Ӿ��� |
A | NaOH | Ar | SO2 |
B | K2SO4 | ʯī | S |
C | CH3COONa | ˮ�� |
|
D | Ba(OH)2 | ���ʯ | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���㽭ʡ�߶������в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
Ϊ������NH4+Ũ����Cl-��Ũ�ȱ�Ϊ1:1����Һ������NH4Cl��Һ�м����������HCl��������NaCl�������İ�ˮ��������NaOH����ȷ����
A���٢� B���� C���ۢ� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ӱ�ʡ������ѧ�ڵ��Ĵε��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��Һ�к��нϸ�Ũ�ȵ�NH4+��F-��HSO3-��K+�����ӣ������м�������NaClO�������Һ��������Ŀ�϶����ӵ��ǣ� ��
A��F- B��ClO- C��HSO3- D��NH4+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ������ѧ�ڵ�����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ�Ͻ���X��Y��Z��W���ֽ�����ɣ������Ͻ����������ֻ��Z��Y���ܽ⣻�����Ͻ����ڳ�ʪ�Ŀ����У�����ֻ����Z�Ļ���������úϽ�����������X����Һ�����Һ��ͨ��ʱ���ֽ�������������ʽ������Һ�У�����������ֻ����X�������ֽ����Ļ��˳����
A��Y��Z��W��X B��Z��Y��W��X C��W��Z��Y��X D��X��Y��Z��W
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ������ѧ��12���ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��Һ�к���HCO3-��SO32-��CO32-��CH3COO-4�������ӣ��������м���������Na2O2����Һ������Ũ�Ȼ������ֲ������
A��CO32- B��SO32- C��CH3COO- D��HCO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�갲��ʡ�߶������в��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��:��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
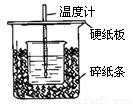
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2�������ͼ����ʾ��װ�ý������飬��õ��к�����ֵ (�ƫ��ƫС����Ӱ�족)����3��ʵ���и���60 mL 0.50 mol��L-1�����50 mL 0.55 mol��L-1NaOH��Һ���з�Ӧ����
��2����ʵ����ȣ������к���_________ (���ȡ�����ȡ�)��
��: ��1���������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6��,��������ɵñ�ϩ����֪��C3H8(g) === CH4(g)��HC��CH(g)��H2(g) ��H1=+156.6 kJ��mol��1
CH3CH��CH2(g)=== CH4(g)�� HC��CH(g ) ��H2=+32.4 kJ��mol��1
��C3H8(g) === CH3CH��CH2(g)��H2(g) ��H = kJ��mol��1��
��2��������ʱ���£� ����ȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪
����ȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪ ��ȫ����������Ӧ�ų�
��ȫ����������Ӧ�ų� ���������Ȼ�ѧ����ʽ�ǣ� ��
���������Ȼ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫��ͷ��ɽ��ѧ��һ��12���¿���ѧ���������棩 ���ͣ�������
ijK2CO3��KHCO3�Ļ���ﹲ6.14 g�����ȷֽ����������Ϊ5.52 g��
��1����ԭ�������K2CO3��������������д��������̣���������ȷ��0.1%��
��2������ԭ���������200 mLˮ�����Һ������Һ����μ���1.0 mol��L-1��ϡ���ᡣ�������ϡ��������Ϊ mLʱ��ʼ�������壻������50 mLϡ����ʱ�������������ڱ�״���µ����Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���㽭ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���л�ѧʵ��ʱӦǿ����ȫ��ʶ������������ȷ����
A���������Ż�ʱʹ����ĭ��������
B�����Թܼ���̼�����ƹ���ʱʹ�Թܿ���ֱ����
C��Ũ���ὦ��Ƥ����ʱ������ϡ����������Һ��ϴ
D������������������ʵ���ҵ��������ʱ��Ӧ������������Ʒۣ�Ȼ������ɨ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com