
����Ŀ������ѧһѡ��3�����ʽṹ�����ʡ�
±��Ԫ�ص������������ʷ�Ӧ�γɶ��ֻ������������ѧ���ʽṹ�����ʵ����֪ʶ�ش�
(1)д����̬��ԭ�ӵļ۵����Ų�ʽ___________��
(2)±��Ԫ�صĺ�������������ǿ����____(д��ѧʽ)����������ӵ����幹��Ϊ_________��
(3)�Ƚ��������±������۵�ͷе㣬������仯���ɼ�ԭ��_________��
GeCl4 | GeBr4 | GeI4 | |
�۵�/�� | -49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
(4)��֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6(![]() )��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
)��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
(5)��֪��Ԫ����������������ֻ��1�����ӡ�����������ԭ�ӹ�����������ӵ�Ԫ��M�γɵ�һ�ֻ����������������ͼ��ʾ��
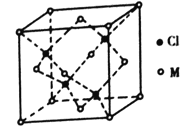
�ٸû�����Ļ�ѧʽΪ_______����֪��������a=0.542 nm���˾�����ܶ�Ϊ_____g/cm3��(д������ʽ����Ҫ��������������ӵ�����ΪNA)
���𰸡� 5s25p3 HClO4 �������� GeCl4��GeBr4��GeI4���ۡ��е��������ߡ�ԭ���Ƿ��ӽṹ���ơ���Է����������������Ӽ������������ǿ < 11:1 CuCl ![]() ��
��![]()
��������(1)��ĺ˵����Ϊ53����̬��ԭ�ӵĵ����Ų�ʽΪ[Kr]4d105s25p5����۵����Ų�ʽ5s25p3��
(2)���ȵ����и�����������ǿ�Ǻ���������ǿ�ᣬ��ѧʽΪ��HClO4�������������������ԭ��ΪsP3�ӻ���û�й¶Ե����������幹��Ϊ�������壻
(3)���±���ﶼ�Ƿ��Ӿ��壬���Ӽ�ͨ�����Ӽ���������ϣ����������ṹ���Ƶķ��Ӿ��壬��Է�������Խ���Ӽ�������Խǿ���۷е�Խ�ߣ�������Է���������GeCl4��GeBr4��GeI4���ʷе㣺GeCl4��GeBr4��GeI4��
(4)H5IO6(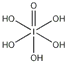 )�к���5���ǻ��⣬Ϊ��Ԫ�ᣬ�����ǻ���ԭ��1����HIO4ΪһԪ�ᣬ����1���ǻ��⣬�����ǻ���ԭ��3�����������ԣ�H5IO6��HIO4��H5IO6��������11��������Ϊ1�����ߵĸ�����Ϊ11:1��
)�к���5���ǻ��⣬Ϊ��Ԫ�ᣬ�����ǻ���ԭ��1����HIO4ΪһԪ�ᣬ����1���ǻ��⣬�����ǻ���ԭ��3�����������ԣ�H5IO6��HIO4��H5IO6��������11��������Ϊ1�����ߵĸ�����Ϊ11:1��
(5)���ݾ����ṹ�������и������ÿ�������к���ͭԭ�Ӹ���Ϊ��8��![]() +6��
+6��![]() =4����ԭ�Ӹ���Ϊ4����ѧʽΪ��CuCl��1mol�����к���4molCuCl��1mol����������ΪM(CuCl)��4����������a=0.542nm�������ܶ�Ϊ
=4����ԭ�Ӹ���Ϊ4����ѧʽΪ��CuCl��1mol�����к���4molCuCl��1mol����������ΪM(CuCl)��4����������a=0.542nm�������ܶ�Ϊ![]() =
=![]() ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����˫ѡ��![]() gͭ������ŨH2SO4����ʱ��ȫ��Ӧ���ڱ�״��������
gͭ������ŨH2SO4����ʱ��ȫ��Ӧ���ڱ�״��������![]() L���壬��ԭ��H2SO4�����ǣ���BC����
L���壬��ԭ��H2SO4�����ǣ���BC����
A. ![]() mol B.
mol B. ![]() mol C.
mol C. ![]() g D.
g D. ![]() g
g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ֱ���ʢ�Т���ɫʯ����Һ ��NaOH��Һ ��Ʒ����Һ �����Ը��������Һ���Թ���ͨ��SO2���塣
��1���Թܢ��е�����___________������Ӧ�Ļ�ѧ����ʽ�ǣ�_________________��
��2���Թܢ��з�����Ӧ�Ļ�ѧ����ʽ�ǣ�_____________________�����ͨ�������SO2��������Ӧ�Ļ�ѧ����ʽΪ��_______________________________��
��3���Թܢ��е�����________���罫����SO2��ĸ���Һ���ȣ�����______________��
��4���Թܢ��е�����____________________��
��5������ʵ���У�SO2���ֳ��������������ʵ���___________�����Թܱ�ţ���ͬ����SO2���ֳ�Ư���Ե���___________��SO2���ֳ���ԭ�Ե���___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֺ���Ԫ�ص�����֮����ת����ϵ��ͼ��ʾ��

��1��д��ͼʾ��Ӧ�ļ������ʵĻ�ѧʽ��B________��C________��D________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ����B��A��______________________________��
��B��E��_____________________________________________________��
��3��д��������Һ�з�Ӧ�����ӷ���ʽ��D��C___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ȡ������һ�ֲ�����֬���ᱻ��Ϊ���Իƽ𡱣�����ֻ��̼���⡢������Ԫ�ء��������ܶ��Ǽ����24 ��������̼����������Ϊ81.25%������һ��������ֻ��һ���Ȼ�����ͨ������ȷ���á��Իƽ𡱵ķ���ʽ��______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ˮ����ʱ��ͨ���Ƚ�����ˮɹһ��ʱ�����ע����ף�Ŀ���� ( )
A�����ˮ�� B������ˮ�������ĺ���
C ����ȥˮ�������Ĵ����� D����������ɱ��ˮ�е�ϸ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧһѡ��5���л���ѧ������
�л���B����̼��������Һ��Ӧ����������̼�����䱽���ϨD�ȴ���ֻ��2�֣�GΪC��F��һ�������°���1��1��Ӧ���ɵĸ߷��ӻ�������������ת����ϵ�ش�
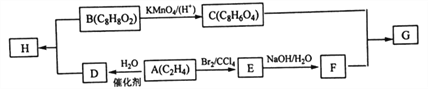
��֪��![]()
(1) D�����������ŵ�����Ϊ______��E������Ϊ_______��
(2) G�Ľṹ��ʽΪ ______��E��F�ķ�Ӧ����Ϊ______��
(3)�����й�˵����ȷ����_____(�����)��
A.D���Է���������Ӧ
B.1molC������2molNaHCO3��Ӧ
C.C��F��һ���������¿��Է�Ӧ���ɻ�״������
D.H�����е�̼ԭ�Ӳ�������ͬһ��ƽ����
(4)B��D��Ӧ����H�Ļ�ѧ����ʽΪ_______________��
(5)ͬʱ������������B��ͬ���칹�干��____�֣������������칹����
���ܷ���������Ӧ�ں��б����ṹ�����ڼ���������ˮ��
���к˴Ź��������г���4��壬�ҷ����֮��Ϊ3 : 2 : 2 : 1_______(д���ṹ��ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����HCl����������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.20g/cm3��������1L 1mol/L��ϡ���ᡣ�ش������й����⡣��������������裺
��1�����㣺����ȡ36.5%��Ũ��������Ϊ________������
��2����ȡ������Ͳ��ȡ����Ũ���Ტע�뵽250mL�ձ��У�
��3���ܽ⣺___________________________________________________����ȴ��
��4��ת�ƣ���Һ��:_________________________________________________��
��5��ϴ�ӣ�__________________________________________________________��
��6�����ݣ�__________________________________________________________��
��7��ҡ�ȣ��Ǻ�����ƿ���������ߵ���ҡ�ȣ�
��8�����أ������úõ�ϡ���ᵹ���Լ�ƿ�У������ñ�ǩ����ǩ��Ҫע��______________��
��9�������������²��������õ�����Ũ���ǡ�ƫ�ߡ�������ȡ����ǡ�ƫ�͡���
������Ͳ��ȡŨ�����������ˮϴ��Ͳ�������Һת������ƿ�У�____________��
������ƿ��������������ˮ��__________________��
��û�н�ϴ���ձ��Ͳ���������Һת������ƿ�У�___________��
�ܶ��ݶ���ʱ����������ƿ�Ŀ̶��ߣ�________________��
�ݶ���ҡ�Ⱥ�������ƿ��Һ����ڿ̶��ߣ��ּ�ˮ��________________��
��δ��ȴ��ת�ƶ��ݣ�_____________________��
����ϴ������ƿ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ե��з�̪��Һ��������Һ����������ɫ�������(����)
A��AlCl3��Һ��������AlCl3
B��CH3COONa��Һ����
C����ˮ�м�������NH4Cl����
D��������Һ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com