
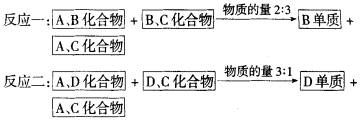
��ش�
(1)DԪ������������ˮ����Ļ�ѧʽ��________��B���ʵĵ���ʽ��________��B��Cԭ�Ӱ뾶��С��ϵΪ________��
(2)д�����㡰��Ӧ�����Ļ�ѧ��Ӧ����ʽ________��
(3)��������Ԫ����ɵĻ�������(����Ԫ�ض���)����ͬŨ����pH��С�Ļ�����Ļ�ѧʽ��_______________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��

| 3 |
| ||
| 3 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
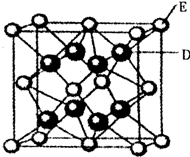 ��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�
��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�| 5.2��10-22 |
| �� |
| 5.2��10-22 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
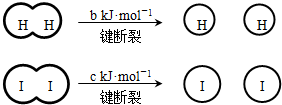 ��a��b��c�������㣩
��a��b��c�������㣩| A����Ӧ�������������������������� | B���Ͽ�1mol H-H����1mol I-I�������������ڶϿ�2mol H-I���������� | C���Ͽ�2mol H-I����������ԼΪ��c+b+a��kJ | D�����ܱ������м���2mol H2��2mol I2����ַ�Ӧ��ų�������С��2a kJ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com