
����Ŀ���������ݰ�½�ȵ���ʢ�����ӣ�����Ҷ��������ƽ������Ѫ���١�ֹʹ�����ã���һ��������ҩ�ġ�������Ҷ���Գɷֻ�ͪ�����ʵ���ȡ���õļ������ܼ���ȡ�����������������ᾧ������������ȡ���ȡ����й��ڷ����ᴿʵ�������Ͳ�����ȷ����
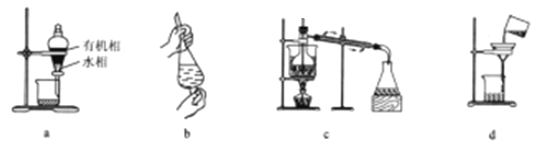
A. ��װ��a��Һ���ų�ˮ����ٴӷ�Һ©���¿ڷų��л���
B. ����b:�����ʹֲ�Ʒ������Ҷ�з���������л��ܼ���ȡ���е���Ч�ɷ��������в�ʱ����������ʹ©��������ų�
C. ��װ��c����������������л��ܼ������ȥ�õ�����Ҷ��Ч�ɷֻ�ͪ������
D. �ᾧ������װ��d���������ܼ����뿪
���𰸡�B
��������A����Һʱ��Ϊ����Һ�����»�϶���Ⱦ���²�Һ����¿�©�����ϲ�Һ����Ͽڵ�����ѡ��A����B. ����b:�����ʹֲ�Ʒ������Ҷ�з���������л��ܼ���ȡ���е���Ч�ɷ��������в�ʱ����������ʹ©��������ų���ѡ��B��ȷ��C. ��װ��c��������������¶ȼƲ��������¶ȣ���Ӧ����������ƿ֧���ܿ����룬ѡ��C����D������ʱ�����ò�����������ѡ��D����ѡB��


 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ũ����μӵ�ʢ�ж������̷�ĩ��Բ����ƿ�У�ʵ���ҿ�����ͼװ����ȡ��ˮ�Ȼ�������֪��FeCl3���������տ����е�ˮ�������⣩����ش���������:

��1��ʢ��Ũ�������������Ϊ_______________��
��2����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��3��Cƿ�е��Լ���________________��
��4��������D�з�����Ӧ�Ļ�ѧ����ʽΪ______________________________����Ӧ������__________________________��
��5�������E��ʢ�м�ʯ�ң���������___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯�������Ź㷺����;����ش�
��1����¯������Ŀǰ�ձ���õ�ұ�����ķ�������֪��
FeO(s)+CO(g)![]() Fe(s)+CO2(g) ��H=a kJ��mol-1
Fe(s)+CO2(g) ��H=a kJ��mol-1
Fe2O3(s)+CO(g)![]() 2FeO(s)+CO2 ��H=b kJ��mol-1
2FeO(s)+CO2 ��H=b kJ��mol-1
��ҵ��������ұ�������Ȼ�ѧ����ʽΪ_________________________________��
��2��Fe(NO3)3��Һ�����ڿ�ʴ�������ù����д���Fe2+��Fe3+֮���ת����
�ٸ÷�Ӧ�����ӷ���ʽΪ_________________________________��
��T ��ʱ����0.5mol��L-1 Fe(NO3)3��Һ�м�������Ag����Һ��c(Ag+)�뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ1��ʾ����Һ����仯���Բ��ƣ���
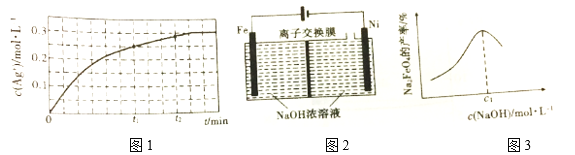
t1ʱ����Һ��c(Fe3+)=__________��t1��t2ʱ���淴Ӧ����v��1______v��2���������������=��������Ϊ_________________________________��
��T ��ʱ���������Ϊ100 mL��0.5mol��L-1 Fe(NO3)3��Һ��0.4mol��L-1 Fe(NO3)2��Һ��0.6mol��L-1 AgN03��Һ��Ϻ��ټ���6.0g Ag��һ��ʱ���Ag������________(�������С�����䡱)��
��ʵ���������ͬ�����£�FeCl3��Һ��Fe(NO3)3��Һ������ʴ��������Ϊ__________��
��3��Na2FeO4����Ҫ��ˮ����������ǿ���Խ������ȶ�������ͼ2��ʾװ���Ʊ�����֪�����ʱ�����Ũ�ȹ��ߣ�������������ɫ���ʡ�
�ٵ��ʱ������������Һ�ļ���__________(�������С�����䡱)��
�ڵ��ʱ��Na2FeO4�IJ�������ʼ�����c(NaOH)�Ĺ�ϵ��ͼ3��ʾ��c(NaOH)=c1 mol��L-1ʱ��Na2FeO4��������ԭ��Ϊ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����һ���¶��£��ݻ�Ϊ0.5L���ܱ������У���һ�����Ķ��������Ͷ������������Ϻ�����Ӧ��SO2(g)��NO2(g) ![]() NO(g) ��SO3(g)
NO(g) ��SO3(g)
������ƽ����ϵ��ͨ������O2��ƽ�� ________�ƶ����������������������������������� NO��Ũ�Ƚ�_______������������������������������������c��NO����c��NO2��֮�� ________������������������������������������
��2������ʱ�����е�N2��O2�ᷴӦ����NO����Ⱦ������N2(g)��O2(g)===2NO(g)�������Է����е�����¶���__________����[��֪�÷�Ӧ����H����180.50kJ/mol����S����247.7 J/(mol��K)]
��3��25 ��ʱ����a mol��L��1��ˮ��0.01 mol��L��1����������ϣ���Ӧƽ��ʱ��Һ��c(NH![]() )��c(Cl��)������Һ��________(������������������������)�ԡ��ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb��________��
)��c(Cl��)������Һ��________(������������������������)�ԡ��ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb��________��
��4���ö��Ե缫���M(NO3)x��ˮ��Һ��������������a gʱ����������ͬʱ����b L����(��״��)���Ӷ���֪M�����ԭ������Ϊ________________����a��b��x��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з����е�����ԭ���ӻ������������ͬ����( )
A. CO2��SO2 B. CH4��NH3 C. BeCl2��BF3 D. C2H2��C2H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��R��X��Y��Z�����ڱ�ǰ������Ԫ����ԭ��������������R�������к�������Ԫ����X��Y��Z����Ԫ�صĻ�̬ԭ���ڲ������ܼ������������ӡ�XԪ�صĻ�̬ԭ�ӵĺ�����ӷֲ��������ܼ��ϣ���ÿ���ܼ����Ų�����ͬ�ĵ�������Y��Xͬ��������Y��X�Ļ�̬ԭ����δ�ɶԵ�������ͬ��Z����������X����������2��Z�Ļ�̬ԭ�������ֻ��1�����ӡ��ش���������:
��1��ZԪ�صļ۵����Ų�ʽΪ____________��
��2��R2Y����Z�������������γ�һ�ָ�������[Z(R2Y)4]2+��[Z(R2Y)4]2+�ļ��ι�����______����[Z(R2Y)4]2+��������γɵ�����Һװ���Թ�������μ��백ˮֱ������,���ֵ�ʵ��������______________��
��3��X��R���γ�һ�ֻ�ѧʽΪX3R4�IJ��ȶ��������һ�ֻ����м�����X3R4������3��Xԭ�ӳ�ֱ���������м��Xԭ�ӵ��ӻ��������Ϊ_____�ӻ�����X3R4�����е�4��Rԭ�ӱ�����4����ͬ����Ԫ�ص�ԭ��ȡ��ʱ���γɵ��·���___(����������������������)���ԡ�
��4���������з�ʽ�У�ͨ��������Z�ľ���Ķѻ���ʽ��______(�����)��Z�ľ�����,Zԭ�ӵ���λ��Ϊ________��
A.ABCABCABC B.ABABABABAB C.ABBAABBA D.ABCCBCABCCBA
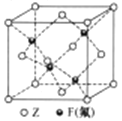
��5��Z��F(��)�γɵ�һ�ֻ�����ľ����ṹ����ͼ��ʾ���������ܶ�Ϊag.cm-3����Z��F(��)�������Ϊ______pm(�����ӵ�������NA��ʾ,�г��������ʽ,���û���,1pm=1.0��10-12m)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡһ�����ʵ���Ũ�ȵ�NaOH��Һ100mL��Ȼ������ͨ��һ������CO2���壬�õ���ҺA����A����λ�������0.1mol/L��HCl��Һ��������CO2�����������״����������HCl��Һ�����֮���ϵ��ͼ��ʾ��ͨ������ش�
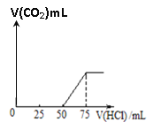
��1��A��Һ�е�����Ϊ_________����ѧʽ���������ʵ���֮����_______��
��2��ͨ��CO2�������__________________����״������
��3��NaOH��Һ�����ʵ���Ũ��______________________��
��4������ͨ���CO2����Ϊ112mL, �����õ���Һ��λ�������0.1mol/L��HCl��Һ������������CO2�����������״�����Ĺ�ϵͼ________________����Ӧ���ֹؼ�������ݣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧʵ��С������ͼװ����ȡ���ռ���������İ�������̽���������й����ʡ�
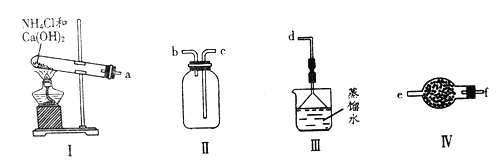
�ش���������:
��1��д������װ��I��ȡ�����Ļ�ѧ����ʽ_________________________________��
��2���������������������ӵ�˳��:a��___________________________��d(����ĸ��ű�ʾ)��
��3��ʵ����װ��III������________________��
��4��װ��IV������������Ϊ________��ʢװ���Լ�Ϊ______________��
��5���ڼס��Ҳ��������зֱ��ռ��������Ȼ��⣬����ͼװ�ý���ʵ�顣������K,�۲쵽��ʵ��������_____________________________��
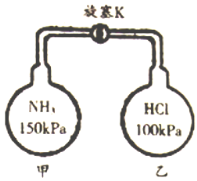
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com